Nature Knows and Psionic Success
God provides
Outsmarting Brain Scams: Your 4-Step Plan

Sebastian Voortman/Pexels Open Google, your favorite social media platform, or any tabloid magazine, and you’re certain to hear about the latest hacks to feel amazing for the rest of your life. Whether it’s a supplement, superfood, novel device, app, or even a pharmaceutical, we’re constantly inundated with the promise that the only thing standing between where we stand now and clearer thinking, better focus, or improved mood is just a quick purchase away. Yet the vast majority of these pitches don’t deliver even a fraction of their promise. Here’s how to avoid wasting your time and money on what doesn’t work, and what to do instead. 1. Avoid the sensationalized
The bigger the promise, the higher the chance it won’t deliver. Whether it’s rapid weight loss or a pill that will boost your IQ overnight, any pitch that assures you of an incredible, unbelievable outcome (especially if it doesn’t require any work on your end) is almost certainly a gimmick or other fallacy. Though there is great data for a number of brain-boosting strategies, these are nearly exclusively interventions that require commitment and deliver more reasonable results that compound over time. 2. Skip the quick fix
There are few red flags as important as the “quick-fix” scheme. From money-making scams to overnight brain-boosting supplements, anything that guarantees a complete turnaround in brain function in the blink of an eye is relatively sure to be misleading. Scientific research absolutely supports the role of a number of strategies to help improve brain function but these are long-term plays, not instant outcomes. 3. Ignore the fluff and ask for the science
It’s easy to be lulled into buying a product when there’s an “expert” behind it. It may be even more compelling when our favorite celebrity swears by a device, or when a bunch of people on social media have given a five-star vote of confidence . But while these plays may make sense for a new piece of furniture or garment, they shouldn’t really apply when we’re talking about brain health interventions. The truth is that the majority of “brain-boosting” products on the market are based on non-existent or incredibly limited data. That matters a lot when we’re promised something like better mood or clearer thinking. 4. Choose the sustainable, healthy habits that are most linked to better brain health
Most brain products may be suspect, but this doesn’t mean powerful, effective tools don’t exist. Instead, the things that are best known to support better brain function today and for the rest of your life are simple steps that are largely unrelated to purchasing any fancy or “breakthrough” product.
The Basics That Matter Most:
> Quality sleep: Good sleep is a rapid and powerful intervention for better brain health including sharper focus, better memory , and better mood. And, quality sleep is linked to better long-term brain health as well.
Regular exercise: Physical activity is one of the most studied and consistently proven ways to enhance brain function today and for years to come. It may also protect against brain diseases like Alzheimer’s.
Eating real food: Eating a typical diet has been shown to increase risk for brain issues, but consuming a diet rich in minimally processed foods, (especially fruits, vegetables, nuts, seeds, and healthy fats like olive oil) may help decrease the odds of mental health issues and dementia .
Stress management : Chronic stress is well known to damage the brain. Mindful practice, time in nature, breathing exercises, and working with a qualified mental health professional can counter stress and help keep your brain in top form.
Continuous learning: Research shows that people who keep learning over their lifespans are at lower risk for developing dementia. Keep your brain sharp by learning something new and pushing your brain’s abilities each day.
Social connection: Compelling data shows that the more socially connected we feel, the better our health is. This may be especially true for brain health. Call a loved one, set a coffee date, or make plans with friends.
New research on deep brain stimulation shows how objective biomarkers could improve depression treatment

Deep brain stimulation can alleviate treatment-resistant depression for some patients. Read more It can be challenging to create a treatment plan for depression. This is especially true for patients who aren’t responding to conventional treatments and are undergoing experimental therapies such as deep brain stimulation.
For most medical conditions, doctors can directly measure the part of the body that is being treated, such as blood pressure for cardiovascular disease. These measurable changes serve as an objective biomarker of recovery that provides valuable information about how to care for these patients.
On the other hand, for depression and other psychiatric disorders, clinicians rely on subjective and nonspecific surveys that ask patients about their symptoms. When a patient tells their doctor they are experiencing negative emotions, is that because they are relapsing in their depression or because they had a bad day like everyone does sometimes? Are they anxious because their depression symptoms have lessened enough that they are experiencing new feelings, or do they have some other medical problem independent of their depression?
Each reason may indicate a different course of action, such as altering a medication, addressing an issue in psychotherapy or increasing the intensity of brain stimulation treatment.
Advertisement
We are neuroengineers . In our study, newly published in Nature, we identified potential biomarkers for deep brain stimulation that could one day help guide clinicians and patients when making treatment decisions for those using this approach to alleviate treatment-resistant depression. Biomarker for depression
Clinical depression does not respond to available therapies in a significant number of patients. Researchers have been working to find alternative options for those with treatment-resistant depression , and many decades of experiments have identified specific brain networks with abnormal electrical activity in those with depression.
This notion of depression as abnormal brain activity rather than a chemical imbalance led to the development of deep brain stimulation as a depression treatment: a surgically implanted, pacemaker-like device that delivers electrical impulses to certain areas of the brain. Studies testing this technique have found that it can decrease depression severity over time in most patients.
Our research team wanted to find specific changes in brain activity that could serve as a biomarker that objectively measures how well deep brain stimulation is helping patients with depression. So we monitored the brain activity of 10 patients receiving deep brain stimulation for severe treatment-resistant depression over six months.
At the end of six months, 90% of the patients responded to the therapy — defined by a reduction of symptoms by at least a half — and 70% were in remission, meaning they no longer met the criteria for clinical depression.
To identify a potential biomarker, we developed an algorithm that looked for patterns in brain activity changes as patients recovered. The algorithm was based on data from six out of the original 10 patients who had usable data from the experiment. We found that there are coordinated changes in different frequencies present in the electrical activity within the area of the brain being stimulated. Using these patterns, the algorithm was able to predict whether someone was in a stable recovery with 90% accuracy each week.
Interestingly, we observed some parts of this pattern moved in the opposite direction later in stimulation therapy compared with the patterns at the start of therapy. This finding provides evidence that the long-term recovery is due to the brain adapting to the stimulation in a process called plasticity rather than as a direct effect of the stimulation itself.
We also saw other potential biomarkers worth investigating further.
For example, abnormalities in brain imaging taken before implanting the electrodes in specific parts of the brain correlated with how sick each patient was. This could provide clues about what’s causing depression in some people, or help develop imaging methods to determine who might be a good candidate for deep brain stimulation.
For another example, we found that the facial expressions of patients changed as their brains changed over the course of their treatment. While physicians often report this anecdotally, quantifying these changes may provide a way to develop objective markers of recovery that incorporate a patient’s behavior with their brain signals.
Because the results of our study are based on a small sample of patients, it’s important to further investigate how broadly they can be applied to other patients and newer deep brain stimulation devices. Improving decision-making for depression
Clinical depression is a debilitating condition that causes significant personal and societal suffering . It is one of the largest contributors to the overall disease burden of many countries. Despite the many approved treatments available, nearly 30% of the 8.9 million U.S. adults taking medications for clinical depression continue to have symptoms.
Deep brain stimulation is one of the alternative therapies for treatment-resistant depression that researchers are investigating. Studies have shown that deep brain stimulation can offer effective and long-term relief for some patients.
Although deep brain stimulation is an approved treatment for other conditions like Parkinson’s disease , it remains an experimental therapy for treatment-resistant depression. While the results from small experimental studies have been positive, they have not been successfully replicated in large-scale, randomized clinical trials necessary for approval from the U.S. Food and Drug Administration.
Finding an objective biomarker that measures recovery in depression has the potential to improve treatment decisions. For example, one patient in our study had a relapse after several months of remission. Were a biomarker available at the time, the clinical team would have had warning that the patient was relapsing weeks before standard symptom surveys showed that anything was wrong. Such a tool could help clinicians intervene before a relapse becomes an emergency.
4 Hidden Sources of Brain Toxins in Your Home

Alexander Dummer/Unsplash Having a healthy, well-functioning brain is a great priority for all of us. And each day we get to make decisions that either vote for brain health, or against it. Many of the variables that contribute to the health of our brains are located within the walls of our homes. All too often, we’re unaware of hidden brain toxins lurking in our kitchens, bathrooms, and bedrooms. Specifically, we’re not thinking about the risk posed by the air we breathe each day. Here are three of the top hidden sources of airborne brain toxins in our homes and how to help decrease the risk they pose. 1. Air freshening products
In grocery stores and drugstores, it’s common to find an aisle with large groups of “air freshener” products. From scented candles to “fresh-smelling” sprays to “natural” plug-in air diffusers, we’re told through marketing to use these products to cover up stinky odors and get our homes smelling great again. There’s a big problem here: These products typically contain a host of chemicals that when released may directly damage our health, including our brain health. Air freshening products are packed with molecules called “volatile organic compounds” or VOCs, which are potent air pollutants and include known carcinogens like formaldehyde and benzene. In addition, scented candles and aerosol sprays may release high levels of particulate matter that is a known brain toxin. If you chose to freshen your air, consider simmering some spices and herbs in water on your stove. Additionally, look for unscented cleaning supplies and candles. 2. Cooking smoke
Cooking at home is a great way to make sure you’re feeding your brain quality ingredients. Yet research shows that indoor cooking is one of the top contributors to indoor air pollution, which in turn is a known risk factor for worse brain function. You can help mitigate this risk by keeping windows open when cooking, using a hood or other ventilation strategies, cooking less high-heat smoky dishes, and using an air purifier. There’s a debate over gas stoves right now, and while there are pros and cons for gas stoves, electric and induction stoves may be better options purely from the air-pollution perspective. 3. New furniture, paints, and chemicals
We all get excited thinking about a new home, renovations, a new piece of furniture, or a new paint color. Yet research shows that all of these things may increase our exposure to “off-gassing” of VOCs and other air pollutants. Exposure to indoor VOCs is a known health risk, and has been shown to correlate with worsened cognitive testing. Risk for off-gassing appears to be a more significant risk for certain groups of purchases including new carpets, pressed/engineered wood furniture, new foam mattresses, and paint/liquid chemicals. Here are a few strategies to help minimize risk: Store paints and other chemicals or items covered in chemicals outside your house.
Limit purchases of new potentially high off-gassing home items to one or a couple items at a time.
Choose real wood for furniture when possible.
Increase ventilation in rooms with new furniture or other high off-gassing products.
Look for certifications on your furniture, paint, and mattresses that designate lower or no emissions.
Buy used furniture.
4. Incense
Incense has been used for a wide variety of purposes in homes and in buildings for thousands of years. It’s usually made from a mix of elements including wood powder, adhesive and chemicals that provide scent . When incense is burned, it creates a host of tiny particles as well as potentially toxic gasses like formaldehyde and benzene, none of which are doing your overall or brain health any favors. This is especially relevant as it relates to PM 2.5, tiny pollutants generated by incense that are known to spike when incense is burned. PM 2.5 exposure is now believed to increase risk depression , dementia , and more. If you choose to use incense, minimize the amount burned, and use good ventilation.
Study in mice shows brain is ‘rewired’ during pregnancy to prepare for motherhood

Credit: Martha Sexton/public domain Researchers at the Francis Crick Institute have shown that pregnancy hormones “rewire” the brain to prepare mice for motherhood.
Their findings, published in Science , show that both estrogen and progesterone act on a small population of neurons in the brain to switch on parental behavior even before offspring arrive. These adaptations resulted in stronger and more selective responses to pups.
It is well known that while virgin female rodents do not show much interaction with pups, and mothers spend most of their time looking after young. It was thought that hormones released when giving birth are most crucial for this onset of maternal behavior.
But earlier research also showed that rats who have given birth by cesarean section, and virgin mice exposed to pregnancy hormones , still display this maternal behavior, suggesting that hormone changes already during pregnancy may be more important.
In the current study, the researchers found that female mice indeed showed increased parental behavior during late pregnancy, and that exposure to pups wasn’t necessary for this change in behavior.
They found that a population of nerve cells (galanin-expressing neurons) in an area of the brain called the medial preoptic area (MPOA) in the hypothalamus, associated with parenting, was impacted by estrogen and progesterone.
Brain recordings showed that estrogen simultaneously reduced the baseline activity of these neurons and made them more excitable, whereas progesterone rewired their inputs, by recruiting more synapses (sites of communication between neurons).
Making these neurons insensitive to hormones completely removed the onset of parental behavior during pregnancy. Mice failed to show parental behavior even after giving birth, suggesting there is a critical period during pregnancy when these hormones take effect.
While some of these changes lasted for at least a month after giving birth, others seem to be permanent, suggesting pregnancy can lead to long-term rewiring of the female brain.
Jonny Kohl, Group Leader of the State-Dependent Neural Processing Laboratory at the Crick, said, “We know that the female body changes during pregnancy to prepare for bringing up young. One example is the production of milk, which starts long before giving birth. Our research shows that such preparations are taking place in the brain, too.
“We think that these changes, often referred to as ‘baby brain,’ cause a change in priority—virgin mice focus on mating, so don’t need to respond to other females’ pups, whereas mothers need to perform robust parental behavior to ensure pup survival. What’s fascinating is that this switch doesn’t happen at birth—the brain is preparing much earlier for this big life change.”
Rachida Ammari, postdoctoral fellow at the Crick, and first author along with Ph.D. student Francesco Monaca, said, “We’ve demonstrated that there’s a window of plasticity in the brain to prepare for future behavioral challenges. These neurons receive a large number of inputs from elsewhere in the brain, so now we’re hoping to understand where this new information comes from.”
The researchers believe the brain may also be rewired in a similar way during pregnancy in humans, as the same hormonal changes are expected to impact the same areas of the brain. This could influence parental behavior alongside environmental and social cues.
Provided by The Francis Crick Institute
Electrifying Recovery: How Brain Stimulation Lights the Path Out of Depression

Researchers have identified a unique biomarker in the brain that signifies recovery from treatment-resistant depression, utilizing deep brain stimulation and artificial intelligence to understand and enhance treatment outcomes. A breakthrough study reveals a unique biomarker in the brain that tracks recovery from severe depression, using innovative deep brain stimulation and AI techniques.
A team of leading clinicians, engineers, and neuroscientists has made a groundbreaking discovery in the field of treatment-resistant depression published online in the journal Nature on September 20.
By analyzing the brain activity of patients undergoing deep brain stimulation (DBS), a promising therapy involving implanted electrodes that stimulate the brain, the researchers from Emory University School of Medicine, Georgia Institute of Technology, and the Icahn School of Medicine at Mt. Sinai identified a unique pattern in brain activity that reflects the recovery process in patients with treatment-resistant depression. This pattern, known as a biomarker, serves as a measurable indicator of disease recovery and represents a significant advance in treatment for the most severe and untreatable forms of depression.
The team’s findings offer the first window into the intricate workings and mechanistic effects of DBS on the brain during treatment for severe depression. How DBS Works and Its Impact
DBS involves implanting thin electrodes in a specific brain area to deliver small electrical pulses, similar to a pacemaker. Although DBS has been approved and used for movement disorders such as Parkinson’s disease for many years, it remains experimental for depression. This study is a crucial step toward using objective data collected directly from the brain via the DBS device to inform clinicians about the patient’s response to treatment. This information can help guide adjustments to DBS therapy, tailoring it to each patient’s unique response and optimizing their treatment outcomes. Monitoring and Artificial Intelligence in Treatment
Now, the researchers have shown it’s possible to monitor that antidepressant effect throughout the course of treatment, offering clinicians a tool somewhat analogous to a blood glucose test for diabetes or blood pressure monitoring for heart disease: a readout of the disease state at any given time. Importantly, it distinguishes between typical day-to-day mood fluctuations and the possibility of an impending relapse of the depressive episode.
The research team used artificial intelligence (AI) to detect shifts in brain activity that coincided with patients’ recovery.
The study, funded by the National Institutes of Health Brain Research Through Advancing Innovative Neurotechnologies, or the BRAIN Initiative, involved 10 patients with severe treatment-resistant depression, all of whom underwent the DBS procedure at Emory University. Advanced Techniques and Findings
The study team used a new DBS device that allowed brain activity to be recorded. Analysis of these brain recordings over six months led to the identification of a common biomarker that changed as each patient recovered from their depression. After six months of DBS therapy, 90% of the subjects exhibited a significant improvement in their depression symptoms and 70% no longer met the criteria for depression.
“This study demonstrates how new technology and a data-driven approach can refine DBS therapy for severe depression, which can be debilitating,” says John Ngai, PhD, director of the BRAIN Initiative. “It’s this type of collaborative work made possible by the BRAIN Initiative that moves promising therapies closer to clinical use.”
The high response rates in this study cohort enabled the researchers to develop algorithms known as “explainable artificial intelligence” that allow humans to understand the decision-making process of AI systems. This technique helped the team identify and understand the unique brain patterns that differentiated a “depressed” brain from a “recovered” brain. Insights From Experts
“The use of explainable AI allowed us to identify complex and usable patterns of brain activity that correspond to a depression recovery despite the complex differences in a patient’s recovery,” explains Sankar Alagapan PhD, a Georgia Tech research scientist and lead author of the study. “This approach enabled us to track the brain’s recovery in a way that was interpretable by the clinical team, making a major advance in the potential for these methods to pioneer new therapies in psychiatry.”
Helen S. Mayberg, MD, co-senior author of the study, led the first experimental trial of subcallosal cingulate cortex (SCC) DBS for treatment-resistant depression patients at Emory University in 2003, demonstrating it could have clinical benefit. In 2019, she and the Emory team reported the technique had a sustained and robust antidepressant effect with ongoing treatment over many years for previously treatment-resistant patients.
“This study adds an important new layer to our previous work, providing measurable changes underlying the predictable and sustained antidepressant response seen when patients with treatment-resistant depression are precisely implanted in the SCC region and receive chronic DBS therapy,” says Mayberg, now founding director of the Nash Family Center for Advanced Circuit Therapeutics at Icahn Mount Sinai. “Beyond giving us a neural signal that the treatment has been effective, it appears that this signal can also provide an early warning signal that the patient may require a DBS adjustment in advance of clinical symptoms. This is a game changer for how we might adjust DBS in the future.”
“Understanding and treating disorders of the brain are some of our most pressing grand challenges, but the complexity of the problem means it’s beyond the scope of any one discipline to solve,” says Christopher Rozell, PhD, Julian T. Hightower Chair and Professor of Electrical and Computer Engineering at Georgia Tech and co-senior author of the paper. “This research demonstrates the immense power of interdisciplinary collaboration. By bringing together expertise in engineering, neuroscience and clinical care, we achieved a significant advance toward translating this much-needed therapy into practice, as well as an increased fundamental understanding that can help guide the development of future therapies.” Observations and Further Research
The team’s research also confirmed a longstanding subjective observation by psychiatrists: as patients’ brains change and their depression eases, their facial expressions also change. The team’s AI tools identified patterns in individual facial expressions that corresponded with the transition from a state of illness to stable recovery. These patterns proved more reliable than current clinical rating scales.
In addition, the team […]
Neurons aren’t the only cells that make memories in the brain, rodent study reveals

Blue micrograph shows the activity of neurons in the hippocampus of a rat as shown by white flecks Neurons, the brain cells responsible for relaying chemical and electrical messages , have long been considered the key players in memory formation — but new research in rodents suggests that the cells may have an unsung-but-crucial collaborator.
The findings could have implications for research into memory and associated diseases, such as Alzheimer’s disease.
Found in the walls of tiny blood vessels called capillaries, the collaborators, called pericytes , are crucial for regulating blood flow in the brain, forming blood vessels, controlling the entry of immune cells into the central nervous system , and constituting and maintaining the blood-brain barrier , a thin border of cells that allows only select molecules to move between the brain and blood.
In addition to these jobs, pericytes also work with neurons to form and store long-term memories, according to a study published Monday (Oct. 2) in the journal Neuron .
“We now have a firmer understanding of the cellular mechanisms that allow memories to be both formed and stored,” Cristina Alberini , senior study author and a professor of neural science at New York University (NYU), said in a statement . “It’s important because understanding the cooperation among different cell types will help us advance therapeutics aimed at addressing memory-related afflictions,” she added.
In the new study, the authors looked at a protein called insulin-like growth factor 2 (IGF2), whose production surges in the hippocampus , a key region of the brain for making long-term memories, after learning. For example, there’s an uptick in IGF2 after an animal is trained to be fearful of scenarios that they’ve come to associate with a mild electric shock to the foot.
In mice and rats, the researchers found that pericytes produce most of this IGF2 in the hippocampus. This production seemed to be triggered by the activity of nearby neurons; when starting to form memories, neurons in the hippocampus send each other a flurry of chemical messages and the channels of communication between those cells begin to grow stronger . The researchers aren’t yet sure how this activates the nearby pericytes, but it does seem that the neurons kick off the memory-making process.
In other experiments, the researchers stopped pericytes from producing IGF2 but didn’t stop other types of cells from doing so, such as neurons and connective tissue-making cells called fibroblasts . This not only hindered the rodents’ ability to make long-term memories — for example, of objects they’d been trained to recognize — but also blocked the action of genes that normally switch on in neurons during memory making.
Taken together, these experiments suggest that pericytes need to produce IGF2 for neurons to successfully make long-term memories.
Going forward, the authors want to explore whether IGF2 engages with other types of cells in the brain and whether similar collaborations between neurons and pericytes happen elsewhere in the brain. In their paper, they wrote that it would be valuable to understand whether this mechanism involves all of the pericytes in the hippocampus or only a selective group of them.
The findings may improve our understanding of brain diseases that involve the loss of long-term memories, such as Alzheimer’s disease , which has also been linked to dysfunctioning pericytes .
“This work connects important dots between the newly discovered function of pericytes in memory and previous studies showing that pericytes are either lost or malfunction in several neurodegenerative diseases, including Alzheimer’s disease and other dementia,” study co-author Benjamin Bessières , a postdoctoral researcher at NYU, said in the statement.
But more research is needed, particularly in humans.
“Our study provides a new view of the biology of memory — though more research is needed to further understand the roles of pericytes and the vascular system in memory and its diseases,” Alberini said in the statement.
Emily is a health news writer based in London, United Kingdom. She holds a bachelor’s degree in biology from Durham University and a master’s degree in clinical and therapeutic neuroscience from Oxford University. She has worked in science communication, medical writing and as a local news reporter while undertaking journalism training. In 2018, she was named one of MHP Communications’ 30 journalists to watch under 30. ( emily.cooke@futurenet.com )
Scientists discover neurons that act as brain ‘glue’ to recall memories
Credit: Pixabay/CC0 Public Domain Scientists have discovered new insights into how our brain stores episodic memories—a type of long-term, conscious memory of a previous experience—that could be critical to the development of new neuroprosthetic devices to help patients with memory problems, like Alzheimer’s disease and dementia.
The new study—led by the University of Glasgow, in collaboration with the University of Birmingham and University of Erlangen—used special electrodes, implanted directly into the brains of epilepsy patients requiring surgery, to allow scientists to observe the activity of individual neurons in the hippocampus region of the brain.
The hippocampus is a challenging area to study, due to its location deep within the brain, yet this area is critical for our memory, acting as the librarian to the memory library in our brain.
During everyday life , the hippocampus helps our brain take snapshots of experiences, to store them for later use, by housing neurons that hold a record of what happened when and where. Like a librarian, the hippocampus directs us to where memories are stored in the brain’s neocortex, akin to guiding us to the right bookshelf without knowing the book’s content.
When we learn something new, the information is first processed in the neocortex and then linked to specific groups of neurons in the hippocampus, before forming what’s called a neural assembly that represents a memory.
In this study, scheduled for publication in Nature Human Behaviour and currently available on the bioRxiv preprint server, led by Dr. Luca Kolibius, patients were asked to form memories of pairs or triplets of images. Images were composed of animals, faces of famous people and popular places (e.g., a seagull paired with Brad Pitt and the Taj Mahal). Patients used stories to link the different images to form one coherent memory and were later asked to recall the images when being presented with one image (e.g., a seagull).
The research team, led by Professor Simon Hanslmayr, analyzed the firing rates of neurons during the memory formation and retrieval stage, and identified neurons that increased their firing rates for individual events during memory formation and recall. These neurons—which they called Episode Specific Neurons—appear to reactivate this neural assembly when we recall a memory, triggering the rest of the assembly and rekindling the memory in full.
The researchers believe the neurons discovered in this study represent the “glue” that will keep a record of all the elements of an episodic memory together, i.e., what happened where and when, and therefore has potential to inform the development of neural prosthesis to help treat memory problems in patients. For instance, a device could be developed that stimulates these neurons during memory retrieval to aid recall of memories.
Professor Hanslmayr, University of Glasgow, said, “We are incredibly excited by our findings because neurons that behave in such a way have been speculated to exist in the human hippocampus for a long time, but this is the first time we actually observed such neurons. Our next step will be to test whether stimulation of these neurons can trigger the recall of memories, which would demonstrate causality. Thereby, this research could lead to the development of devices which can help with those suffering memory-related health conditions.”
Dr. Kolibius says, “The neurons we discovered are, in essence, acting as our brain’s memory guide, helping us access and retrieve our cherished memories, like a librarian leading us to the right book on the shelf; so, to discover more about how they work is both exciting and important.”
Provided by University of Glasgow
Groundbreaking research focuses on brain health monitoring, early detection of neurodegenerative diseases
Associate Professor at the University of Oulu Teemu Myllylä, a leading expert in biomedical engineering, recently discussed his team’s groundbreaking work in brain health monitoring and early detection of neurodegenerative diseases at the recent 6G-enabled sustainable society event. Their research focuses on wearable technologies and direct sensing techniques for neurohydrodynamics, which have the potential to revolutionize the early diagnosis and treatment monitoring of Alzheimer’s disease.
Neurodegenerative diseases (NDDs) remain a significant public health challenge, causing a high burden of disability and mortality worldwide. Recent figures show that NDDs are the second leading cause of death worldwide, resulting in 276 million disability-adjusted life years (DALYs) and 9 million deaths annually. Of these, Alzheimer’s disease (AD) is particularly severe. It causes 28.7 million DALYs and 2.4 million fatalities each year. Nearly 500,000 new cases of Alzheimer’s disease are expected to be diagnosed each year. Parkinson’s Disease, in turn, is linked to 3.6 million years of DALYs and leads to the deaths of 211,000 people.
Myllylä and his team are pushing the boundaries to measure brain activity using wearable and wireless technology. Ultimately, they hope that their research could one day result in a breakthrough of applications capable of detecting the early signs of Alzheimer’s disease or other neurodegenerative diseases before any symptoms appear.
Their device, Glymphometer, utilizes light to monitor brain fluid dynamics and possible disorders it causes in the brain, providing real-time assessment of a patient’s brain health. For instance, the device is capable of distinguishing AD patients from healthy controls based on a 5-minute measurement. The next phase of their research involves determining how early they can detect the risk of Alzheimer’s disease or other neurodegenerative diseases before any symptoms appear, which would be a significant breakthrough in the field.
Early intervention is crucial for successful therapeutics and underscores the importance of developing new technologies and tools to detect and treat neurodegenerative diseases as early as possible. Multidisciplinary cooperation across organizations and countries
In Oulu, Finland, Myllylä’s team works in the Health Sciences and Technology research unit. They conduct multidisciplinary research with various departments at the University of Oulu, including the Faculty of Medicine and Information Technology and Electrical Engineering (ITEE), the Center for Wireless Communications (CWC), as well as with VTT (Technical Research Centre of Finland). The possibility of performing preclinical research in Oulu jointly with the Oulu Biocenter (BCO) and Oulu University Hospital is also highly important.
To validate their methods and further develop the technology, the team closely collaborates with various medical centres and institutes worldwide. At present, they start to collect significant amounts of brain data from patients and healthy controls in Finland, USA, Germany and the Netherlands using the developed wireless brain monitoring technology. This will also open up the possibility to study the use of 5G and, in the future, 6G in emerging healthcare applications and to streamline data collection, as their aim is to gather data from hundreds of patients each year. By analysing this extensive dataset, they aim to develop machine-learning-based methods to calculate index values for the risk of neurodegenerative diseases. The G Index: A personalized approach to brain health monitoring
A central component of Myllylä’s research is the so-called Glymphatic system activity index (G Index), a measure of brain clearance activity. Early results indicate that this index is impacted by elements such as sleep quality and daily tasks. The goal of his research is for individuals to be able to monitor their G Index and make adjustments to their lifestyles to reduce the risk of developing brain disorders. For maintaining optimal cognitive health, monitoring the G Index twice daily could eventually become a common practice among people 40-70 years old, as this age range is most critical when observing one’s cognitive health.
Myllylä’s team is working with partners in the United States and the Netherlands to study how daily activities affect brain clearance activity. By understanding how these activities impact the G Index, they hope to offer recommendations on lifestyle changes that could improve brain health and reduce the risk of neurodegenerative diseases. Future of early NDD detection
Professor Myllylä’s research offers a promising glimpse into the future of brain health monitoring at home and the early detection of neurodegenerative diseases. As wearable technologies and direct sensing techniques continue to advance, we may soon see a world where individuals can take charge of their brain health, making informed decisions to minimize their risk of developing debilitating conditions like Alzheimer’s disease.
In the future, Myllylä believes his team can develop a system for continuous brain health monitoring that can detect and alert individuals when their G Index starts to deviate from its normal range. This could offer people enough time to make lifestyle changes and/or start clinical therapies before any irreversible brain damage has been done. Could this be a game-changing path in the fight against neurodegenerative diseases?
Source:
The University of Oulu
Be the first to rate this article
Posted in: Medical Research News | Medical Condition News
Psychedelics plus psychotherapy can trigger rapid changes in the brain: New research is untangling how
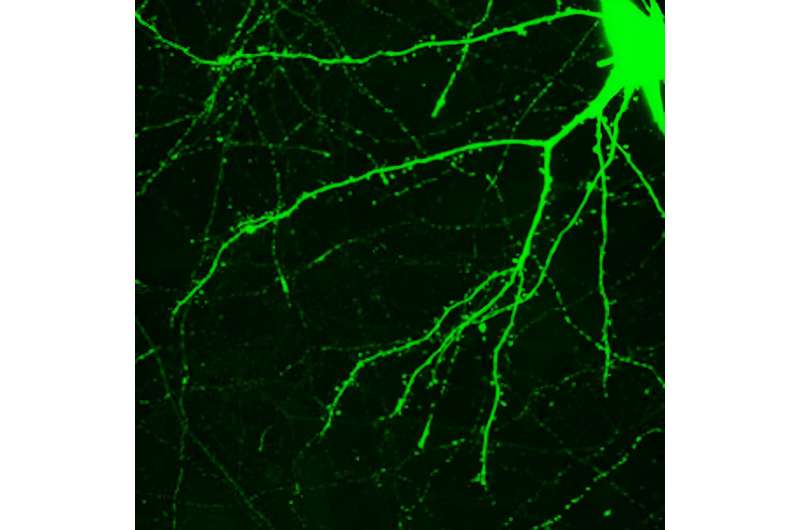
Neuronal spines are the little bumps along the spreading branches of a neuron. Credit: The human brain can change —but usually only slowly and with great effort, such as when learning a new sport or foreign language, or recovering from a stroke. Learning new skills correlates with changes in the brain , as evidenced by neuroscience research with animals and functional brain scans in people. Presumably, if you master Calculus 1, something is now different in your brain. Furthermore, motor neurons in the brain expand and contract depending on how often they are exercised—a neuronal reflection of “use it or lose it.”
People may wish their brains could change faster—not just when learning new skills, but also when overcoming problems like anxiety, depression and addictions.
Clinicians and scientists know there are times the brain can make rapid, enduring changes. Most often, these occur in the context of traumatic experiences , leaving an indelible imprint on the brain.
But positive experiences, which alter one’s life for the better, can occur equally as fast. Think of a spiritual awakening , a near-death experience or a feeling of awe in nature .
Social scientists call events like these psychologically transformative experiences or pivotal mental states . For the rest of us, they’re forks in the road. Presumably, these positive experiences quickly change some “wiring” in the brain.
How do these rapid, positive transformations happen? It seems the brain has a way to facilitate accelerated change. And here’s where it gets really interesting: Psychedelic-assisted psychotherapy appears to tap into this natural neural mechanism. Psychedelic-assisted psychotherapy
Those who’ve had a psychedelic experience usually describe it as a mental journey that’s impossible to put into words. However, it can be conceptualized as an altered state of consciousness with distortions of perception, modified sense of self and rapidly changing emotions. Presumably there is a relaxation of the higher brain control, which allows deeper brain thoughts and feelings to emerge into conscious awareness.
Psychedelic-assisted psychotherapy combines the psychology of talk therapy with the power of a psychedelic experience. Researchers have described cases in which subjects report profound, personally transformative experiences after one six-hour session with the psychedelic substance psilocybin, taken in conjunction with psychotherapy. For example, patients distressed about advancing cancer have quickly experienced relief and an unexpected acceptance of the approaching end. How does this happen? Tiny spines along a neuron’s branches are a crucial part of how one neuron receives a message from another. Credit: Edmund S. Higgins Research suggests that new skills, memories and attitudes are encoded in the brain by new connections between neurons—sort of like branches of trees growing toward each other. Neuroscientists even call the pattern of growth arborization .
Researchers using a technique called two-photon microscopy can observe this process in living cells by following the formation and regression of spines on the neurons. The spines are one half of the synapses that allow for communication between one neuron and another.
Scientists have thought that enduring spine formation could be established only with focused, repetitive mental energy. However, a lab at Yale recently documented rapid spine formation in the frontal cortex of mice after one dose of psilocybin. Researchers found that mice given the mushroom-derived drug had about a 10% increase in spine formation. These changes had occurred when examined one day after treatment and endured for over a month. A mechanism for psychedelic-induced change
Psychoactive molecules primarily change brain function through the receptors on the neural cells. The serotonin receptor 5HT, the one famously tweaked by antidepressants , comes in a variety of subtypes. Psychedelics such as DMT, the active chemical in the plant-based psychedelic ayahuasca , stimulate a receptor cell type , called 5-HT2A. This receptor also appears to mediate the hyperplastic states when a brain is changing quickly.
These 5-HT2A receptors that DMT activates are not only on the neuron cell surface but also inside the neuron. It’s only the 5-HT2A receptor inside the cell that facilitates rapid change in neuronal structure. Serotonin can’t get through the cell membrane , which is why people don’t hallucinate when taking antidepressants like Prozac or Zoloft. The psychedelics, on the other hand, slip through the cell’s exterior and tweak the 5-HT2A receptor, stimulating dendritic growth and increased spine formation.
Here’s where this story all comes together. In addition to being the active ingredient in ayahuasca, DMT is an endogenous molecule synthesized naturally in mammalian brains. As such, human neurons are capable of producing their own “psychedelic” molecule, although likely in tiny quantities. It’s possible the brain uses its own endogenous DMT as a tool for change—as when forming dendritic spines on neurons—to encode pivotal mental states. And it’s possible psychedelic-assisted psychotherapy uses this naturally occurring neural mechanism to facilitate healing. A word of caution
In her essay collection ” These Precious Days ,” author Ann Patchett describes taking mushrooms with a friend who was struggling with pancreatic cancer. The friend had a mystical experience and came away feeling deeper connections to her family and friends. Patchett, on the other hand, said she spent eight hours “hacking up snakes in some pitch-black cauldron of lava at the center of the Earth.” It felt like death to her.
Psychedelics are powerful, and none of the classic psychedelic drugs, such as LSD, are approved yet for treatment. The U.S. Food and Drug Administration in 2019 did approve ketamine, in conjunction with an antidepressant, to treat depression in adults. Psychedelic-assisted psychotherapy with MDMA (often called ecstasy or molly) for PTSD and psilocybin for depression are in Phase 3 trials.
Provided by The Conversation
This article is republished from The Conversation under a Creative Commons license. Read the original article .
These Are All the Vitamins for Brain Health You Need in Your Diet

These Are All the Vitamins for Brain Health You Need in Your Diet “Hearst Magazines and Yahoo may earn commission or revenue on some items through these links.”
[table-of-contents] stripped
When it comes to keeping your brain healthy as you age, your diet plays a big role. Eating a variety of foods is critical to getting the vitamins and nutrients your brain needs to keep performing at its best. But the best vitamins for brain health may help.
Research has found that “certain nutrients, flavonoids, unsaturated fats, and omega-3 fatty acids are associated with slower cognitive decline and reduced risk of dementia ,” says Puja Agarwal, Ph.D. , a nutritional epidemiologist and assistant professor in the Department of Internal Medicine at Rush Medical College in Chicago.
Meet the Experts: Puja Agarwal, Ph.D. , a nutritional epidemiologist and assistant professor in the Department of Internal Medicine at Rush Medical College in Chicago; Mirella Díaz-Santos, P.h.D., an assistant professor in the Mary S Easton Center for Alzheimer’s Disease Research; Robin Foroutan, M.S., R.D.N. , a functional dietitian; Gill Livingston, M.D. , a professor of psychiatry at University College London.
Although eating whole foods is the best way to get those brain-boosting nutrients. supplements for brain health can be a helpful option in specific circumstances. “In general, supplements aren’t often useful for brain health unless you have a deficiency in certain nutrients, which happens but is rare,” says Gill Livingston, M.D. , a professor of psychiatry at University College London whose research focuses on dementia prevention, intervention, and care.
So, which vitamins support brain health ? And how can you get more of those essential vitamins into your diet? Ahead, experts share everything you need to know. Vitamins for brain health
Omega-3 fatty acids
If you’ve ever wondered why fatty fish like salmon and tuna are always touted as part of a healthy diet, here’s one reason: They’re high in omega-3 fatty acids, a type of unsaturated fat that has a brain-protecting anti-inflammatory effect and is a building block of cell membranes in the brain. Shop Now Nordic Naturals Ultimate Omega amazon.com $32.49 Nordic Naturals More Omega-3s have also been linked to lower levels of beta-amyloid, a type of protein found in the brains of people with Alzheimer’s-related damage. “Omega-3 fatty acids easily penetrate the blood-brain barrier and are essential for the brain’s structure and functioning,” explains Dr. Agarwal.
Foroutan adds there has been some research that indicates high doses of omega-3 fatty acids after a concussion or other traumatic brain injury may have protective effects on lasting damage
Where to find it: Besides fatty fish, good sources of omega-3s include nuts and seeds and some fortified foods such as eggs and yogurt. If you’re someone who doesn’t eat seafood often, check with your doctor about taking an omega-3 supplement if bloodwork indicates you’re deficient, says Mirella Díaz-Santos, P.h.D., an assistant professor in the Mary S Easton Center for Alzheimer’s Disease Research at UCLA’s Department of Neurology and Women’s Alzheimer’s Movement partner. Vitamin E
This vitamin functions as an antioxidant in the body, and it protects cells from oxidative stress , a type of damage caused by free radicals (unstable molecules in the body), even in the brains of people with Alzheimer’s disease. The brain is particularly susceptible to oxidative stress, which increases during aging and is a major contributor to cognitive decline .
Vitamin E is also anti-inflammatory, which helps to keep DNA healthy and replicating correctly while maintaining the structure of healthy brain cell membranes, says Robin Foroutan, M.S., R.D.N. , a functional dietitian.
Where to find it: Vitamin E can be found in dark leafy greens, avocado, red bell pepper, asparagus, mango, pumpkin, and nuts and seeds. B Vitamins
When it comes to brain health, focus on the three B’s: vitamins B6, B12, and B9 ( folate ). “These three types of B vitamins are necessary for the brain’s normal functioning ,” says Dr. Agarwal, “and any deficiency in them may increase the risk of memory loss and other forms of cognitive decline.”
The reason: These vitamins help boost the production of neurotransmitters , or brain chemicals, that deliver messages between the brain and body.
Increasing your B12 by taking a supplement may also be helpful with memory loss as you age because it’s a very common nutrient for older people to develop a deficiency in, notes Díaz-Santos.
Where to find them: Beans are one of the best sources of B vitamins across the board. You can find B6 in bananas, oranges, papaya, cantaloupe, tuna, salmon, poultry, and dark leafy greens. Folate is found in broccoli, greens, whole grains, eggs, peanuts, and sunflower seeds.
Vitamin B12 is found solely in meat and fish products; for vegans and vegetarians, nutritional yeast and fortified whole grains are a good way to get your supply. People on a plant-based diet do have a much higher risk of a true B12 deficiency, so talk to your doctor or dietitian about whether or not a B12 supplement is right for you. Vitamin C
This antioxidant is known for its immunity powers, but vitamin C and other flavonoids also support the brain , potentially by taming brain-damaging inflammation.
In one study , by Rush University researchers including Dr. Agarwal, people who consumed vitamin C-rich strawberries at least once a week were less likely to develop Alzheimer’s over the course of the nearly 20-year study period.
Where to find it: Get vitamin C in abundance from kiwi, red and green bell peppers, citrus, berries, broccoli, cauliflower, brussels sprouts, and tomatoes. Supplements for brain health
There’s a lot of mixed research and feeling among experts when it comes to taking supplements for brain health. Most experts agree it’s always better to spend your money on nutritious foods, but there are exceptions.
Díaz-Santos says that if you’re someone with an allergy or aversion to a large food group (like seafood or dairy) or your doctor found a deficiency during a blood panel, you may want to consider taking a dietary supplement. Otherwise, a well-rounded diet […]
Memory Block: How Saturated Fats May Hinder Memory Formation in the Aging Brain

A new study suggests that a high-fat diet may impair memory by causing inflammatory effects and issues in cell-signaling management in the brain cells, especially as people age, but the omega-3 fatty acid DHA may help mitigate these effects. The research, focusing on microglia and hippocampal neurons, found that palmitic acid increased inflammation, while DHA protected against this effect but not against the loss of mitochondrial function induced by palmitic acid exposure. New research reveals DHA shields brain cells from fat-related inflammation.
New research suggests several mechanisms through which high-fat foods may impact brain cells, potentially elucidating the association between a high-fat diet and memory decline, particularly in aging.
A study from The Ohio State University conducted in cell cultures indicates that the omega-3 fatty acid DHA could shield the brain from the detrimental effects of an unhealthy diet by reducing fat-triggered inflammation at the cellular level.
Separate experiments using brain tissue from aging mice showed a high-fat diet may lead specific brain cells to overdo cell-signaling management in a way that interferes with the creation of new memories.
The same lab found in an earlier study in aging rats that a diet of highly processed ingredients led to a strong inflammatory response in the brain that was accompanied by behavioral signs of memory loss – and that DHA supplementation prevented those problems.
“The cool thing about this paper is that for the first time, we’re really starting to tease these things apart by cell type,” said senior author Ruth Barrientos, an investigator in Ohio State’s Institute for Behavioral Medicine Research and associate professor of psychiatry and behavioral health and neuroscience in the College of Medicine.
“Our lab and others have often looked at the whole tissue of the hippocampus to observe the brain’s memory-related response to a high-fat diet. But we’ve been curious about which cell types are more or less affected by these saturated fatty acids, and this is our first foray into determining that.”
The study was published recently in the journal Frontiers in Cellular Neuroscience .
For this work, the researchers focused on microglia, cells in the brain that promote inflammation, and hippocampal neurons, which are important for learning and memory. They used immortalized cells – copies of cells taken from animal tissue that are modified to continuously divide and respond only to lab-based stimulation, meaning their behavior may not precisely match that of primary cells of the same type.
Researchers exposed these model microglia and neurons to palmitic acid, the most abundant saturated fatty acid in high-fat foods like lard, shortening, meat, and dairy products, to observe how it affected gene activation in the cells as well as functioning of mitochondria, structures inside cells that have a primary metabolic role of generating energy.
Results showed that palmitic acid prompted gene expression changes linked to an increase in inflammation in both microglia and neurons, though microglia had a wider range of affected inflammatory genes. Pre-treatment of these cells with a dose of DHA, one of two omega-3 fatty acids in fish and other seafood and available in supplement form, had a strong protective effect against the increased inflammation in both cell types.
“Previous work has shown that DHA is protective in the brain and that palmitic acid has been detrimental to brain cells, but this is the first time we’ve looked at how DHA can directly protect against the effects of palmitic acid in those microglia, and we see that there is a strong protective effect,” said Michael Butler, first author of the study and a research scientist in Barrientos’ lab.
When it came to the mitochondria, however, DHA did not prevent the loss of function that followed exposure to palmitic acid.
“The protective effects of DHA might, in this context, be restricted to effects on gene expression related to the pro-inflammatory response as opposed to the metabolic deficits that the saturated fat also induced,” Butler said.
In another set of experiments, the researchers looked at how a diet high in saturated fat influenced signaling in the brains of aged mice by observing another microglial function called synaptic pruning. Microglia monitor signal transmission among neurons and nibble away excess synaptic spines, the connection sites between axons and dendrites, to keep communication at an ideal level.
Microglia were exposed to mouse brain tissue containing both pre- and post-synaptic material from animals that had been fed either a high-fat diet or regular chow for three days.
The microglia ate the synapses from aged mice fed a high-fat diet at a faster rate than they ate synapses from mice fed a regular diet – suggesting the high-fat diet is doing something to those synapses that gives the microglia a reason to eat them at a higher rate, Butler said.
“When we talk about the pruning, or refinement, that needs to occur, it’s like Goldilocks: It needs to be optimal – not too much and not too little,” Barrientos said. “With these microglia eating away too much too soon, it outpaces the ability for these spines to regrow and create new connections, so memories don’t solidify or become stable.”
From here, the researchers plan to expand on findings related to synaptic pruning and mitochondria function, and to see how palmitic acid and DHA effects play out in primary brain cells from young versus aged animals.
Reference: “Dietary fatty acids differentially impact phagocytosis, inflammatory gene expression, and mitochondrial respiration in microglial and neuronal cell models” by Michael J. Butler, Sabrina E. Mackey-Alfonso, Nashali Massa, Kedryn K. Baskin and Ruth M. Barrientos, 10 August 2023, Frontiers in Cellular Neuroscience .
DOI: 10.3389/fncel.2023.1227241
This work was supported by grants from the National Institute on Aging and the National Institute of Dental and Craniofacial Research. Additional co-authors, all from Ohio State, were Sabrina Mackey-Alfonso, Nashali Massa and Kedryn Baskin.
Scientists focus on glymphatic system and its role in sleep, memory consolidation, degenerative illnesses

Credit: Pixabay/CC0 Public Domain When we think of brains, we tend to think of neurons. It’s right there in our word for the study of the brain: neuroscience. But when it comes to certain mysteries of the brain—for example, the role of sleep in memory consolidation, or the genesis of traumatic brain injuries (TBIs) and degenerative neurological disorders such as Parkinson’s disease and Alzheimer’s disease—the answers we seek may lie elsewhere: the glymphatic system.
A waste clearance system for the brain and the nervous system of humans and other vertebrates, the glymphatic system derives its name from the brain’s glial cells, on which it depends, and the lymphatic system, which it resembles functionally. It consists of the pathway including channels called ventricles, the brain’s interstitial spaces—the spaces between the cells in the brain’s gray and white matter—and the perivascular spaces around veins and arteries in the brain.
Scientists at the Johns Hopkins Applied Physics Laboratory (APL) in Laurel, Maryland, are working to advance our understanding of the glymphatic system and the role it plays in sleep, memory consolidation, degenerative illnesses and more. Reawakened interest, 150 years later
“If you go back to neuroimaging all the way back to the 1850s, scientists were primarily interested in imaging the ventricles to better understand pathologies like hydrocephalus [a neurological disorder caused by a buildup of excess cerebrospinal fluid (CSF)],” said Clara Scholl, chief scientist of the Neuroscience Group in APL’s Research and Exploratory Development Department (REDD). “And then neurons were discovered, and the focus shifted away from the ventricles and toward cellular matter in the brain for the next 150 years or so.”
In the past decade, studies with fluorescent tracers in mice led to the discovery of the glymphatic system and its role in sleep and neurodegenerative disease, among other questions. “The field has developed a solid understanding of how this system works through animal models, as well as invasive studies in humans in which they inject dyes into the CSF and then observe sleeping subjects using MRI [magnetic resonance imaging] scanners,” Scholl said.
It’s clear by now that a better understanding of the glymphatic system—how it functions, how to observe and influence its workings—could have a significant and positive impact on human health, in a variety of civilian and military applications. But before that can happen, significant technology gaps will have to be addressed.
“If you have a pathology in your glymphatic system, we have no way to determine that without injecting fluorescent tracers into your CSF or putting you in an MRI scanner while you’re sleeping,” said Scholl, “Or in some conditions, you might have a hole drilled in your skull to relieve the pressure and a sample of your CSF can be taken in the process. But there’s really no noninvasive way to observe or measure CSF flow dynamics in the brain. That’s where APL comes in.” From phantoms to humans
APL’s work in glymphatic system sensing began with an effort to use commercial off-the-shelf sensors to track its role in TBI. Sponsored by Uniformed Services University (USU) and supported by the Congressionally Directed Medical Research Programs (CDMRP), this project is a collaboration with Navy sleep physician J. Kent Werner. The potential they saw using noninvasive, near-infrared spectroscopy sensors (NIRS)—a technology traditionally used for tracking hemodynamic activity (blood flow)—inspired the APL team to extend its capabilities.
Within a relatively short period of time, Scholl and the rest of the APL team, guided by the expertise of REDD optical physicist Joseph Angelo, have applied a specialized form of NIRS, known as frequency domain functional NIRS (FD-fNIRS), to develop noninvasive sensors that can accurately track the activity of the glymphatic system.
To tune FD-fNIRS to observe the complex fluid dynamics of the glymphatic system, the APL team developed “optical phantoms” that model the optical properties of the glymphatic system—an effort described in a publication in IEEE Xplore based on a conference paper. Since that accomplishment, Will Coon, a sleep scientist and neural signals engineer in REDD, has gone on to conduct sleep studies in which glymphatic activity was measured and tracked alongside more traditional metrics associated with sleep, such as electroencephalographic (EEG) activity.
Establishing correspondence between the glymphatic system and the known dynamics of sleep is no small feat, but it’s worth the effort. “There’s good reason to believe we can interact with the glymphatic system,” Coon said. “We know the drivers. We’re starting to see that a particular kind of sleep called slow-wave sleep stimulates blood flow into the brain. And when blood flows in, CSF flows out—that seems to be the driver for much of the glymphatic system’s waste clearance activity.”
Coon continued, “Fortunately, there’s an extensive body of research around slow-wave sleep and memory consolidation —we know ways to interact with and control these slow waves, how to increase them, like using clicks and sounds to stimulate more waves. And if we can do that, it points to possible treatments for the glymphatic system—maybe treatments we can apply preventatively with TBI in order to stave off neurological decline 20 or 30 years down the road.” A cold trail revived
Recently, APL began a collaboration with Michael Smith, a sleep doctor at Johns Hopkins Medicine (JHM) in Baltimore. Smith came across a trio of research papers from the late 1980s that found that cooling delivered at specific moments during sleep could induce extended bouts of slow-wave sleep in human subjects. Those findings were replicated not long ago by several European research teams, which brought them to Smith’s attention.
“This is an exciting discovery, because using sound-based methods, you can only turn one or two slow waves into five or six waves; but this thermal effect has the potential to induce hundreds, maybe even thousands of slow waves, which adds up to significantly more restorative slow-wave sleep ,” Coon explained. “That could be a game-changer for helping aging populations achieve better glymphatic clearance, including healthy people and those with neurological disorders, including Alzheimer’s and Parkinson’s.” Reaching for the STARS: A comprehensive sleep ecosystem
Coon conducts research at APL targeted […]
Unveiling the Brain’s Secrets: Study Resolves “Paradox” in Visual Recognition Memory

A new study clarifies past conflicting observations on visual recognition memory (VRM), showing that increased visual evoked potentials (VEPs) during the recognition of familiar stimuli signal the brain’s rapid identification process, ultimately leading to decreased overall neural activity. Since determining what we observe as new or familiar is essential for prioritizing our attention, neuroscientists have dedicated years to understanding why our brains excel at this task.
During their research, they have encountered seemingly conflicting findings. However, a recent study reveals that these perplexing results are really two sides of the same coin, paving the way for a long-sought understanding of “visual recognition memory” (VRM).
VRM is the ability to quickly recognize the familiar things in scenes, which can then be de-prioritized so that we can focus on the new things that might be more important in a given moment.
Imagine you walk into your home office one evening to respond to an urgent, late email. There you see all the usual furniture and equipment—and a burglar. VRM helps ensure that you’d focus on the burglar, not your bookshelves or your desk lamp.
Data from the paper show a sharp but brief increase in neural activity — a visually evokied potential — when a stimulus pattern is shown to a mouse at about 80 milliseconds (bright orange vertical line). Notably when a stimulus is familiar, activity decreases significantly (cooler colors) after that transient increase. Credit: Bear Lab/MIT Picower Institute
“Yet we do not yet have a clear picture of how this foundational form of learning is implemented within the mammalian brain,” wrote Picower Professor Mark Bear and fellow authors of the new study in the Journal of Neuroscience .
As far back as 1991 researchers found that when animals viewed something familiar, neurons in the cortex, or the outer layer of their brain, would be less activated than if they saw something new (two of that study’s authors later became Bear’s colleagues at MIT , Picower Professor Earl K. Miller and Doris and Don Berkey Professor Bob Desimone).
But in 2003 , Bear’s lab happened to observe the opposite: Mice would actually show a sharp jump in neural activity in the primary visual region of the cortex when a familiar stimulus was flashed in front of the animal. This spike of activity is called a “visually evoked potential” (VEP), and Bear’s lab has since shown that increases in the VEPs are solid indicators of VRM.
The findings in the new study, led by former Bear Lab postdocs Dustin Hayden and Peter Finnie, explain how VEPs increase even amid an overall decline in neural response to familiar stimuli (as seen by Miller and Desimone), Bear said. They also explain more about the mechanisms underlying VRM – the momentary increase of a VEP may be excitation that recruits inhibition, thereby suppressing activity overall.
New understanding
Bear’s lab evokes VEPs by showing mice a black-and-white striped grating in which the stripes periodically switch their shade so that the pattern appears to reverse. Over several days as mice view this stimulus pattern, the VEPs increase, a reliable correlate of the mice becoming familiar with—and less interested in—the pattern. For 20 years Bear’s lab has been investigating how the synapses involved in VRM change by studying a phenomenon they’ve dubbed “stimulus-selective response plasticity” (SRP).
Early studies suggested that SRP occurs among excitatory neurons in layer 4 of the visual cortex and specifically might require the molecular activation of their NMDA receptors.
The lab had seen that knocking out the receptors across the visual cortex prevented the increase in VEPs and therefore SRP, but a follow-up in 2019 found that knocking them out just in layer 4 had no effect. So, in the new study, they decided to study VEPs, SRP, and VRM across the whole visual cortex, layer by layer, in search of how it all works.
What they found was that many of the hallmarks of VRM, including VEPs, occur in all layers of the cortex but that it seemed to depend on NMDA receptors on a population of excitatory neurons in layer 6, not layer 4. This is an intriguing finding, the authors said, because those neurons are well connected to the thalamus (a deeper brain region that relays sensory information) and to inhibitory neurons in layer 4, where they had first measured VEPs.
They also measured changes in brain waves in each layer that confirmed a previous finding that when the stimulus pattern is new, the prevailing brain wave oscillations are in a higher “gamma” frequency that depends on one kind of inhibitory neuron, but as it becomes more familiar, the oscillations shift toward a lower “beta” frequency that depends on a different inhibitory population. A short spike amid a long lull
The team’s rigorous and precise electrophysiology recordings of neural electrical activity in the different layers also revealed a potential resolution to the contradiction between VEPs and the measures of labs like that of Miller and Desimone.
“What this paper reveals is that everybody is right,” Bear quipped.
How so? The new data show that VEPs are very pronounced but transient spikes of neural electrical activity that occur amid a broader, overall lull of activity. Previous studies have reflected only the overall decrease because they have not had the temporal resolution to detect the brief spike. Bear’s team, meanwhile, has seen the VEPs for years but didn’t necessarily focus on the surrounding lull.
The new evidence suggests that what’s happening is that VEP is a sign of the activity of the brain quickly recognizing a familiar stimulus and then triggering an inhibition of activity related to it.
“What I think is exciting about this is that it suddenly sheds light on the mechanism, because it’s not that the encoding of familiarity is explained by the depression of excitatory synapses,” Bear said. “Rather, it seems to be accounted for by the potentiation of excitatory synapses on to neurons that then recruit inhibition in the cortex.”
Even as it advances that understanding of how VRM arises, the study still leaves open questions including the exact circuits involved. For instance, the […]
Aerobic plus strength training could help keep the brain young

Aerobic and strength training could help keep the brain young, a new study suggests. Image credit: Rob and Julia Campbell/Getty Images. Engaging in both aerobic exercise and strength training can improve cognitive performance in populations aged over 80 years, a new study suggests.
Participants who performed only cardio/aerobic exercise fared no better than people who were sedentary at mental acuity tests.
The study underscores the value of being physically active as long as possible as one reaches their later years.
A new study from the McKnight Brain Research Foundation , published in the journal GeroScience , finds that for people aged 80 years or older, a combination of cardio/aerobic exercise and strength training may improve cognition.
The study found that people who combined these two types of exercises exhibited higher cognitive performance than people who were sedentary and people who performed cardio exercise alone.
Individuals who engaged in cardio exercise along with strength training — regardless of duration and intensity — were more mentally agile, quicker at thinking, and also had a stronger ability to shift or adapt their thinking as necessary. What forms of exercise are best for cognitive performance?
The study involved 184 cognitively healthy individuals who were 85 to 99 years old, with a mean age of 88.49 years. Of this group, 98 were women. Their exercise regimens were self-reported, with 68.5% participating in some form of exercise.
Individuals were divided into three groups: people who were sedentary, people who did cardio exercise alone, and people who did both cardio exercise and strength training.
The cognition performance of participants was assessed according to the Montreal Cognitive Assessment battery of tests, designed to measure mild cognitive decline and early dementia signs.
The cardio plus strength training group had the highest overall cognitive performance scores.
The cardio plus strength training group scored significantly better than the sedentary group on coding and symbol search tests.
The cardio plus strength training group also scored significantly better than the cardio-alone group on symbol search, letter fluency, and Stroop Color-Word tests .
The cardio-only group’s test results were the same as the sedentary group. Risks of a sedentary lifestyle
“Aerobic and strength training are clearly helpful for older adults, even at an advanced age,” said Dr. Eric Lenze , professor and chair of psychiatry at Washington University School of Medicine, not involved in the current research.
“It’s not unusual,” noted Dr. Lenze, “to slow down with aging, but some degree of physical activity, like regular walking, is important for maintaining function — staying out of the nursing home!”
“Strength training can add to this benefit by keeping elders able to, for example, get up off the toilet. [Such capabilities] are vital for staying independent,” Dr. Lenze pointed out.
Brain health coach and director of the FitBrain Program at Pacific Neuroscience Institute in Santa Monica, Ryan Glatt , also not involved in the research, warned of the risks associated with a sedentary lifestyle: “The risks of sedentary behavior include sarcopenia (loss of muscle mass), reduced physical functioning, an increased risk of falls and fractures, and cognitive impairment.” Why exercise may support cognitive health
A cross-sectional study such as this looks only for associations. It cannot establish a causal link, such as one between cardio plus fitness and mental acuity. As Dr. Lenze put it, “the authors themselves were careful to describe this as exploratory.”
“It’s not clear how helpful [the exercises] are for improving cognitive function like memory, though,” Dr. Lenze added.
He noted, nonetheless, that both types of exercise “would be expected to improve brain health broadly by improving insulin sensitivity, reducing risk for heart attacks and strokes, and keeping people overall more active.”
Glatt suggested it may be that the two forms of activity affect different areas of the brain, saying: “Previous research has found that exercise benefits the brain in similar ways. However, certain types of exercise have been found to affect certain brain regions.”
“For example,” said Glatt, “prior research has found that resistance training can benefit the function and structure of the frontal lobe, while aerobic exercise can benefit the function and structure of brain regions responsible for memory, such as the hippocampus.” Cardio vs strength training
Cardio and aerobic exercises are essentially the same exercises viewed from different perspectives. Both increase a person’s heart rate while increasing the amount of oxygen the body uses.
For this reason, cardio/aerobic exercises can improve heart health and lung function.
Examples of cardio/aerobic exercise include walking, running, cycling, and swimming, or the use of cardio equipment such as rowing machines, elliptical trainers, treadmills, and stair climbers.
Strength training , or resistance training, involves causing your muscles to contract against some form of external resistance. Such resistance might be weights, resistance, bands or medicine balls, for example. The goal of strength training is to increase muscle mass and power, gain joint flexibility, and strengthen bones. How to exercise safely later in life For people in their 80s or 90s, care must be taken to avoid injury when exercising. Glatt said: “Everyone, regardless of age, can have different levels of physical functioning. It is therefore important to seek out guidance from a physical therapist or qualified fitness professional.” Dr. Lenze recommended exploring The National Institute on Aging website (NIA) for ideas about exercises that are appropriate for the elderly, although it is still wise to consult a fitness professional before beginning any new exercise program.NIA suggests that older individual: begin slowly with low-intensity exercises properly warm up before and cool down after exercise remain aware of surroundings when outside hydrate — drink water — before, during, and after exercise, even when one does not feel thirsty, as older individuals can be less sensitive to sensations of thirst take care to exercise in appropriate clothes and shoes discuss an exercise plan with your healthcare provider to avoid exacerbating specific health conditions.
10 Natural Remedies for Adult ADHD

adhd These are the top natural remedies for Adult ADHD. iStock If you’ve had difficulty concentrating due to the stress of the past few years, you’re not alone. Occasional disorganization, poor planning, and restlessness are a normal part of life, especially this year. But these symptoms could signal something more serious, like ADHD/Hyperactivity Disorder, which the Cleveland Clinic describes as “a developmental problem characterized by inattention, hyperactivity, and impulsivity.”
Most adults with ADHD had the symptoms as a kid, whether they were diagnosed or not. Many people go through life untreated either through a lack of diagnosis or because they don’t like the side effects of Ritalin and Adderal, the medications commonly used to treat ADHD.
While it’s always important to talk with your doctor about the best treatment plan for you if you have ADHD—or get a diagnosis if you don’t yet have one—there are natural remedies to try. From turning to nature to leaning on routines, here are 10 of them. Adult ADHD treatment
Dr. Jeffrey Ditzzell, DO , a Manhattan-based psychiatrist who specializes in treating mood and anxiety disorders including ADHD says, “Medications can be an aspect of care, but they’re not the entirety of care.”
To decrease the functional load of medications, Dr. Ditzell educates patients on things like nourishing the body at consistent intervals, getting proper sleep, and living an overall fit lifestyle, which includes exercise, hydration, and adding supplements as necessary. This type of mindset coaching not only helps with ADHD but “over the course of a lifetime improves performance.” In his practice, Dr. Ditzell uses the obstacle—what brought them into the office—as an opportunity to begin to improve their lives. He collaborates with ADHD clients to come up with ideas that will help them pursue purpose-driven activities throughout the day. Populating the intervals of the day is profoundly satisfying and calming to the mind, and Dr. Ditzell says that when patients put in consistent effort, they find that their level of performance and mindset is more efficient than they ever dreamed.
Nev Schulman Reveals the Incredible Way Having ADHD Has Helped Him On Dancing With the Stars Natural Remedies For ADHD
1. Make lists
Some people with ADHD can feel overwhelmed by the enormity of a task ahead of them, but breaking it into smaller chunks makes life’s responsibilities seem more manageable. After breaking up larger tasks into more manageable pieces, making lists allows someone to cross items off a list, which leads to a sense of accomplishment, which people living with ADHD often lack if they’re pinging from one thing to another. 2. Streamline routines
Whitney Olivia Wilson , a life skills teacher in treatment programs, says, “I teach kids to come up with streamlined, unwavering routines for mundane tasks like getting out of the house in the morning, times when their minds may check out and go exploring into other concepts. Setting routines reduces anxiety over forgetting or messing up something simple. Students can stick to the routine until the neuropathway in their brain has it hardwired. Eventually, when you start the routine your brain will automatically take you through the process while you enjoy the wonderland that is your wild, creative mind.”
Related: The 5 Best Free Brain Games Training Apps 3. Move your body
Almost everyone with ADHD reports an improvement in symptoms if they move their body. Some prefer vigorous exercise like running or spinning, while others find relief through yoga. Either way, exercise increases breathing, which has a calming effect. One person with ADHD points out that starting the day with exercise works for her because it’s something to cross off the list, which sets her up for a successful day. 4. Create silence and order
Some find that life itself is loud and distracting, so wearing earplugs creates a cocoon-like sense of calm. Life can feel more disorderly to someone with ADHD, so color coding things with neon Post-it notes can help with visual organization, which helps with mental tranquility. 5. Nourish yourself
Maui-based nutritionist Jennifer Dreisch encourages those struggling with ADHD symptoms to investigate food sensitivities and, if necessary, repair a leaky gut. “80% of our neurotransmitters are made in our gut,” Dreisch says, “if it’s not healthy, our brain isn’t healthy.” She also recommends adding omega 3s and vitamin D to the diet and cutting processed sugar. 6. Turn to nature
Getting outside and into nature is great for most of what ails us, including ADHD, and herbs found in nature are also helpful for treating ADHD naturally . Sarah Holden , a Missoula, Montana-based clinical herbalist, recommends adaptogens like American ginseng, Rhodiola, and ashwagandha to “help the body accept and deal with stress hormones and reduce the impact of stress on the body. Through this mechanism, the body uses the herb to help it regulate the fight or flight response.”
Holden also recommends nootropics like Ginkgo biloba to help with attention, focus, concentration, learning, memory, and mental acuity and nervines like Gotu Kola to tone down or calm the nervous system, reducing hyperactivity. 7. Do anything you know helps
People with ADHD do well with structure, and many find relief through proximity. One woman could never get her clothes to the laundry basket, so they ended up on the bathroom floor, creating visual chaos that wasn’t helpful. Putting a laundry basket in the bathroom was a simple change that helped immensely. A man couldn’t remember to take his vitamins, which help with his ADHD, so he started putting them in his coffee pot so when he went to make coffee in the morning he couldn’t miss them. 8. Strategize
Jill Wheeler, MA, LMHC , a psychotherapist and transformational leadership coach, has a toolbox full of strategies she uses to help herself and her clients who struggle with ADHD. For starters, Wheeler sees ADHD as a strength. “We’re hardwired to be distracted,” she says, “It’s what kept us safe from predators, and though life isn’t as demanding as […]
Lightweight system captures brain activity while mice jump

A new lightweight device with a wisplike tether can record neural activity while mice jump, run and explore their environment. The open-source recording system, which its creators call ONIX, overcomes several of the limitations of previous systems and enables the rodents to move more freely during recording.
The behavior that ONIX allows brings to mind children running around in a playground, says Jakob Voigts , a researcher at the Howard Hughes Medical Institute’s Janelia Research Campus in Ashburn, Virginia, who helped build and test the system. He and his colleagues describe their work in a preprint posted on bioRxiv earlier this month.
To understand how the brain creates complex behaviors — such as those found in social interaction , sensory processing and cognition , which are commonly affected in autism — researchers observe brain signals as these behaviors unfold.
Head-mounted devices enable researchers to eavesdrop on the electrical chatter between brain cells in mice, rats and primates. But as the smallest of these animal models, mice present some significant challenges. Current neural recording systems are bulky and heavy, making the animals carry up to a fifth of their body weight on their skulls. Predictably, this slows the mice down and tires them out.
And most neural recording systems use a tether to relay signals from the mouse’s brain to a computer. But this tether twists and tangles as the mouse turns its head and body, exerting torque that the mouse can feel. Researchers must therefore periodically replace or untangle the tether. Longer tethers allow for more time to elapse between changeouts, but the interruptions still affect natural behavior. And battery-powered, wireless systems add too much weight.
Altogether, these challenges inhibit natural behaviors and limit the amount of time that recording can take place, preventing scientists from studying, for example, the complete process of learning a new task.
ONIX features a collection of engineering improvements to address these challenges. It includes a lower-profile design for the headstage, the head-mounted platform that holds the necessary electrical components for neural recordings.
The tether that relays data, supplies power and helps to control the headstage is extremely lightweight: A typical tether may be 1.8 millimeters in diameter, but this “micro-tether” is 0.4 millimeters. Finally, a device called a commuter responds to the mouse’s movements, actively untwisting the tether during recording.
To see how mice behave with ONIX, the researchers built a 3D arena of Styrofoam in hexagonal blocks, cut to different heights, akin to a miniaturized version of the basalt columns of the Giant’s Causeway in Northern Ireland. Five cameras recording from different angles around the arena captured the animals’ head positions, running speeds and locations.
Mice outfitted with the ONIX system and lightweight micro-tether explored faster than mice equipped with a standard system. The total area these mice explored and their head movements were indistinguishable from those of completely unencumbered mice.
During more than seven hours of recording, uninterrupted by tether changes, the mice jumped up to 15 centimeters from one Styrofoam column to another while researchers captured the animals’ neural activity. Mice with the heavier system and tether did not jump at all.
Different parts of the open-access ONIX system can be swapped out for similar components, enabling other researchers to adapt the system to specific experimental needs or even improve upon it as technology advances.
Cite this article: https://doi.org/10.53053/TFKO2184
30 Best Supplements for Focus and Concentration
Branded content. Us Weekly has affiliate partnerships so we may receive compensation for some links to products and services.
In a world inundated with distractions and demands, the pursuit of enhanced focus and concentration has become a paramount endeavor for many. Astonishingly, recent studies indicate that the average attention span has plummeted to just 8 seconds, shorter than that of a goldfish. Furthermore, in a survey conducted in 2021, a staggering 75% of respondents reported struggling with maintaining concentration during daily tasks. The demand for solutions to sharpen our cognitive faculties has never been greater, and in this comprehensive guide, we present the 30 best supplements meticulously selected to help you harness your mental prowess. Whether you’re seeking to boost productivity at work or unlock your full learning potential, our curated list will serve as your roadmap to a more focused and concentrated life. 30 Best Supplements for Focus and Concentration
> Elm & Rye Nootropics Supplements
ONNIT Alpha Brain Premium Nootropic Brain Supplement
Peak Performance Alpha Day Nootropic Energy Supplement
NEURIVA Plus Brain Supplement for Memory and Focus
NEURIVA Destress Brain Supplement for Focus, Concentration & Accuracy
OLLY Ultra Strength Brain Softgels
Healthycell Focus + Recall, Brain Boost Supplement Liquid Gel
Focus Factor Adults Extra Strength
Youtheory Vitamin B12 B6, Daily Energy and Brain Support Supplement
Rae Wellness Focus Drops
Vital Vitamins Brain Supplements for Memory & Focus
Medchoice 10-in-1 Nootropic Brain Supplements: Memory & Focus Supplement with Ginkgo
Lemme Focus Concentration & Brain Health Gummies with Cognizin Citicoline
CocoaVia Memory & Focus Brain Supplement
Nature’s Nutrition Nootropic Brain Focus Memory Supplement Gummy
Force Factor Forebrain Focus Brain Booster
Nature’s Branch 40-in-1 Brain Booster Supplement For Focus, Memory, Clarity, Energy
ONNIT Alpha Brain Black Label Capsule
Nature’s Craft Better Memory and Focus Supplement for Adults
Host Defense, MycoBotanicals Brain Capsules, Promotes Concentration
Nature’s Peak Brain Supplement for Memory and Focus
SAGA Spotlight Natural Brain Boosting Focus Shots for Enhancing Clarity
Fungies Lion’s Mane Mushroom Brain Health Gummies
One a Day Active Focus Supplements Raw Science 5-HTP Focus & Memory Supplement Life Seasons – Focus-R – Concentration and Focus Supplement Dr. Emil Nutrition 2100mg Organic Lions Mane Mushroom Supplement Roman Focus Supplement FUJI ORCHARD Citicoline 250 mg Supplements Purely Optimal Premium Brain Supplement Elm & Rye Nootropics Supplements Nootropics are supplements that can enhance various cognitive functions, including focus and concentration. As we navigate through our increasingly busy lives, our attention spans are often put to the test. That’s where Elm & Rye Nootropics Supplements come in. Their specially formulated supplements contain natural ingredients that are shown to support brain function. By taking Elm & Rye Nootropics Supplements, you’ll promote healthy brain activity that can help you stay focused on the task at hand. Whether you’re studying for an exam, working on a project at the office or simply looking to improve your cognitive abilities, Elm & Rye Nootropics Supplements may help you achieve your goals. ONNIT Alpha Brain Premium Nootropic Brain Supplement The ONNIT Alpha Brain Premium Nootropic Brain Supplement is the perfect addition to your daily routine if you’re someone who needs an extra boost of focus and concentration. This supplement is made with a unique blend of natural ingredients that work together to support mental performance and clarity. It’s specifically designed to improve memory, focus, and overall cognitive function, helping you stay alert and sharp throughout even the busiest of days. If you’re looking for a way to enhance your mental abilities without relying on caffeine or other stimulants, this is the supplement for you. Try it out for yourself and see the difference it can make in your daily life. Peak Performance Alpha Day Nootropic Energy Supplement Are you looking for a way to increase your focus and concentration throughout the day? Peak Performance Alpha Day Nootropic Energy Supplement may be just the boost you need. This energy supplement is specifically formulated to improve cognitive function, providing you with the energy and mental clarity needed to tackle even the most challenging tasks. With a unique blend of natural ingredients, such as caffeine, ginseng, and ginkgo biloba, you can trust that this supplement will deliver the results you’re looking for. Ditch the afternoon slump and achieve peak performance with Alpha Day Nootropic Energy Supplement. NEURIVA Plus Brain Supplement for Memory and Focus If you’re looking for supplements to help boost your focus and concentration, NEURIVA Plus Brain Supplement might be just what you need. This supplement is designed to support brain health, improve memory, and enhance focus and clarity. Its active ingredients, including coffee fruit extract and phosphatidylserine, work together to promote cognitive function and provide long-lasting benefits. Whether you’re a student or a busy professional, NEURIVA Plus Brain Supplement can help you stay on top of your game by providing the cognitive support you need. Try it out and experience the results for yourself! NEURIVA Destress Brain Supplement for Focus, Concentration & Accuracy Modern life can be stressful, and sometimes it can feel like our minds are working overtime just to keep up with the demands of daily life. That’s where NEURIVA Destress Brain Supplement comes in, offering a helpful aid to support focus, concentration and accuracy. This supplement is specifically designed to combat the stresses of modern living, with a carefully chosen blend of natural ingredients such as ashwagandha and phosphatidylserine to help you stay on top of your game. Whether you’re studying for exams, working on a big project, or just trying to keep up with your busy schedule, NEURIVA Destress can help you achieve your goals by enhancing your mental clarity and reducing stress impact. With this powerful brain supplement, you can feel confident and focused, no matter what life throws your way. OLLY Ultra Strength Brain Softgels If you are looking for an effective way […]
Three new projects launched to map neuronal connections in the mouse and macaque brains

A complete map of all the connections in an entire mammalian brain may be in sight. Allen Institute researchers have just launched three new projects to construct large, detailed maps of neuronal connections in sections of the mouse and macaque brains, with an eye toward creating full wiring diagrams of these animals’ brains in the future. These projects are funded by the National Institutes of Health’s Brain Research Through Advancing Innovative Neurotechnologies ® (BRAIN) Initiative.
Allen Institute research teams will use the funding to: map the fine structures and connections in a 10mm 3 piece of the mouse brain using electron microscopy;
apply new, cutting-edge techniques known as BARseq and BRICseq to trace the long-range connections of hundreds of thousands of neurons in the macaque brain; and
scale up techniques that characterize brain cell types by 3D shape, electrical properties, and gene expression to better understand connectivity between different types of cells across the whole mouse brain.
Project 1: Enhancing transmission electron microscopy techniques to visualize brain cell shape and cell-to-cell connection networks of the mouse brain
Researchers will aim to scale and optimize a transmission electron microscopy (TEM) pipeline. The goal will be to use this pipeline to image an entire hemisphere of a mouse brain at 120 nanometer resolution and the cortical basal ganglia thalamic loop (up to 10 mm 3 ) in very fine detail to better understand how the mouse brain functions. Researchers will then assess whether this technology can be used to image an entire mouse brain-;a significant accomplishment that could provide a valuable roadmap for global neuroscience.
Transmission electron microscopy (TEM) is a technique in which a beam of electrons is shot through a tissue sample to create an extremely detailed image. “It’s become more and more clear over the last few years that most computations of the brain are actually happening, not in isolated areas, but in distributed networks that are brain wide; and so if we’re really going to understand how those kinds of computations work, we need to see the whole network, which means we need to see connections across the whole brain,” said Forrest Collman, Ph.D., Assistant Investigator at the Allen Institute.
Associate Investigator Nuno da Costa, Ph.D., notes that the potential impact for the broader scientific community is significant: “Think of it as a ‘Google Maps’ of every road, every house, and every door. If done properly, it’s a contribution that will last forever.”
Project 2: Mapping how brain cells are connected to one another using barcoded connectomics
For this project, scientists will map brain-wide connections by tracing the winding paths that axons and dendrites make as they reach out and connect to other brain cells. Think of these as the long arms and fingers of the brain cell radiating out of the cell body and reaching out to other cells across the entire brain to create networks. Researchers will trace these intricate paths using an innovative technique known as BARseq, which stands for barcoded anatomy resolved by sequencing.
It works by tagging each cell with a unique RNA barcode that makes it stand out in a cell population. By “connecting the dots” between each barcode, you can trace where and how far a brain cell-;namely its axons and dendrites-;extend.
It is much faster and more efficient than other techniques and can be combined easily with other data. We can actually map the whole macaque brain in a few years instead of 100 years. This is the main motivation.” Xiaoyin Chen, Ph.D., Assistant Investigator at the Allen Institute Project 3: Enhancing a Patch-seq pipeline to yield more data, faster results, and linking different datasets to uncover form and function in the whole mouse brain
It is critical to develop tools that link genetically defined cell types to brain-wide circuit diagrams to understand brain function. In this project, researchers will work to link genetic and circuit datasets by scaling and sharing technologies that measure features common to both datasets across the entire mouse brain.
Specifically, this project aims to enhance the Allen Institute’s ability to generate multi-dimensional data, using the Patch-seq method, and to capture the full structure of neurons from whole brain images through automation, machine vision modeling, and advanced computational techniques. Another key aim of the project is to share the tools they develop with the broader researcher community so that experts across the field can contribute to characterizing cell types and circuits across the whole mouse brain.
“We’re using more sophisticated machine learning-based approaches where you can create these deep neural networks to bring the morphological descriptions into alignment with transcriptomics, with the connectome data, and with the long-range projection data,” said Staci Sorensen, Ph.D., Associate Director of Neuroanatomy at the Allen Institute. “So far, we feel pretty excited about the results that we’re getting. I think it will work especially well at cell subclass levels.”
The Brain-Boosting MIND Diet – New Study Uncovers “Exciting” Improvement

The MIND diet study showed improvements in cognition over a three-year period, particularly during the first two years. Both the MIND diet group and the control group, focusing on calorie reduction, saw improvements, suggesting potential benefits from weight loss. The diet developed at RUSH is believed to help maintain brain health.
New research highlights the importance of a long-term commitment to the MIND diet for maximizing the benefits to brain health.
“The benefits within the new study’s three-year clinical trial weren’t as impressive as we’ve seen with the MIND diet observational studies in the past, but there were improvements in cognition in the short-term, consistent with the longer-term observational data,” said lead study author Lisa Barnes, Ph.D., associate director of the Alzheimer’s Disease Research Center at RUSH .
Results from the study, published in The New England Journal of Medicine , showed that within a three-year period, there was no significant statistical difference in change in cognition for participants in the MIND diet group compared to the usual diet control group; both groups were coached to reduce calories by 250 kilocalories per day. However, there was a significant improvement during the first two years of the study.
“What we saw was an improvement in cognition in both groups, but the MIND diet intervention group had a slightly better improvement in cognition, although not significantly better,” Barnes said. “Both groups lost approximately 5 kilograms over three years, suggesting that it could have been weight loss that benefited cognition in this trial.” ‘Exciting’ improvement
This is the first randomized clinical trial designed to test the effects of a diet thought to be protective for brain health, on the decline of cognitive abilities among a large group of individuals 65 years or older who did not have cognitive impairment. The MIND diet has been ranked among the top five diets by U.S. News & World Report annually for the last six years.
“There is established research that shows that a person’s diet affects health,” Barnes said. “The participants in this study had to have sub-optimal diets as determined by a score of 8 or less on a diet screening instrument before the study even began. It is reasonable to think that either they were going to maintain their cognition or decrease the rate of cognitive decline in the future.”
“It was exciting to see that there was an improvement in cognition over the first year or so, but it could have been due to practice effects on the cognitive tests, and we saw it for the control diet as well, which focused on just caloric restriction.”
Previous research by the late Martha Clare Morris, ScD, showed that there was a slower rate of decline among those who ate specific foods. Morris was a nutritional epidemiologist at RUSH and the original principal investigator of the MIND diet study that was funded by a $14.5 million National Institutes of Health grant and involved two clinical sites, RUSH in Chicago and Harvard School of Public Health in Boston.
In 2015, Morris and her colleagues at RUSH and Harvard University developed the MIND diet — which is short for Mediterranean-DASH Intervention for Neurodegenerative Delay — in preparation for the trial. The diet is based on the most compelling research on the foods and nutrients that affect brain health. As the name suggests, the MIND diet is a hybrid of the Mediterranean and DASH (Dietary Approaches to Stop Hypertension) diets. Both diets have been found to reduce the risk of cardiovascular conditions, such as hypertension, diabetes, heart attack, and stroke. In two studies published in 2015, Morris and colleagues found that the MIND diet could slow cognitive decline and lower a person’s risk of developing Alzheimer’s disease significantly, even if the diet was not followed meticulously. The study tracked 604 participants over three years
The latest trial of the MIND Diet for Prevention of Cognitive Decline in Older Persons, was a randomized, Phase III trial that enrolled 604 people who were overweight and had a suboptimal diet and a family history of Alzheimer’s disease. The trial compared two different diet interventions, both of which included dietary counseling with mild calorie restriction of 250 calories per day for weight loss.
Participants of both groups had individualized diet guidelines developed by dietitians, and they received regular phone and in-person consultations, as well as occasional group sessions over the three-year life of the study. Participants were seen five times during the three years to evaluate their mental abilities, blood pressure, diet, physical activity, health conditions, and medication use.
“Both groups of participants got a lot of support and accountability by trained registered dietitians,” said Jennifer Ventrelle, assistant professor in the Departments of Preventive Medicine and Clinical Nutrition and lead dietitian on the MIND diet trial at RUSH.
“The good news is that this helped all participants improve on average, but unfortunately hindered the ability to detect significant differences between the two groups in this relatively short period of time. Current and future research plans to look at people coached to follow the diet in this format compared to individuals following a usual diet in a format closer to usual care such as brief clinical encounters or a self-guided program with less support.”
“By the end of the study, the average weight loss was approximately 5.5% of initial body weight for all participants, exceeding the study target of 3%, the amount recognized as clinically significant to prevent or improve adverse health outcomes,” Ventrelle said.
“The average MIND score at the end of three years for the MIND group was 11.0 and 8.3 for the control group, placing both groups in a therapeutic range to slow cognitive decline and lower the risk for Alzheimer’s disease, according to previous studies. The significant weight loss and improved MIND scores suggest that the control group also improved their diet and may suggest that following the MIND diet at a score of at least 8.3, coupled with at least a 250-calorie reduction to produce weight loss, may improve cognition. More research is needed to confirm this.” Fish, chicken, […]
Musk’s Neuralink to test brain-chip in humans after FDA nod. How it’s meant to work & key concerns

Bengaluru: The US Food and Drug Administration (FDA) has given the green light to Neuralink, a company founded by Elon Musk, to conduct human trials in brain-computer interface (BCI) technology. This technology involves implanting a tiny chip in the brain that can read and interpret neural signals.
Founded in 2016, the company aims to treat brain diseases and eventually perform human enhancement and augmentation, where biological implants theoretically alter the body to improve physical and mental capabilities. It has been conducting trials in animals like monkeys and pigs since 2018.
However, the company has also faced criticism and scrutiny for its treatment of animals and its management practices. The US Department of Agriculture (USDA) even has the company under investigation for violating animal welfare laws through careless testing without safety.
Engineers and researchers have reportedly expressed concerns and criticisms over the Neuralink BCI project, stating that Musk’s demonstrated technology has been in existence since 2002 with other BCI projects and have criticised its ability to enhance brain functions without enough scientific knowledge about it.
His ideas for human enhancement, they said, could create more division in society by increasing inequality.
Last year, the FDA rejected Neuralink’s application for human testing, citing safety concerns over the device’s design and functionality. It raised doubts about the device’s ability to move in the brain without causing damage, to be removed safely, if needed, without brain tissue damage, and to operate with a lithium battery.
This month, the FDA approved human clinical trials for Neuralink.
Neuralink has been very secretive about its work and research, revealing only a few details to the public. In 2021, the company showed a video of a monkey playing a video game called Pong by using only its brain signals to control the cursor.
Neuralink is not the only player in the field of BCI. There are many other researchers and institutions that have been developing and using BCI for various purposes, including restoring vision, movement, and speech in people who have lost these abilities due to injury or disease.
ThePrint explains what BCI is, how it works, and what Neuralink plans to do with it.
Also Read: What’s 5 Eyes, intel alliance Canada ‘lobbied for joint action’ in Nijjar affair, and its take on row What is BCI and how does it work
BCI is a technology that connects the brain with external devices, such as computers or robots, using electrical signals. The human brain produces oscillating patterns of activity, called brainwaves, that reflect different mental states. These brainwaves can be measured by an electroencephalogram or EEG.
BCIs — the development work of which began in the 1960s and 70s — can be invasive or non-invasive, depending on the objective.
Non-invasive BCI uses external equipment that can sense brain signals through an EEG without penetrating the skull, and then use those signals to control external objects. The first non-invasive EEG control of a physical object was performed in 1988.
Two years later, bidirectional control was established, where an external source could modify the brain signal as well. Devices were able to induce a state of expectation in the brain, thus modifying brainwaves as well.
Invasive BCI, which became popular in the 2000s, involves surgically implanting a chip or a fibre into the brain that can directly read and write neural signals.
Many labs and universities have shown monkeys and rats with implants being able to perform tasks on external objects with their neural signals alone, or by just thinking about it.
These neural signals have also been captured by computers, which then decode them and transmit them to motor or movement neurons, recovering mobility in impaired animals and people.
A similar process is used to restore vision, as well as recording typed language output in people without arms and/or spinal cord injuries. Concerns and limitations
BCI is a very expensive and complex technology and has many limitations and dangers.
For example, volunteers in experiments with BCIs have to remain very still, and there are unknown long-term effects of having a chip in the brain. There is also a high risk of misuse or abuse of the technology.
For the future, there are already many ethical and social issues related to BCI. There are concerns around mind reading and lack of privacy, as well as the ability to track an individual and use technology to gain information. It could also lead to greater discord in society through abuse or selective use of the technology.
There are also ethical concerns around informed consent from those who are impaired in communication or are in ‘vegetative states’ (brain dysfunction) or coma, and worries about equitable access.
While today the technology is limited to very basic and experimental benefits, Musk has reportedly stated that he founded Neuralink because he believes artificial intelligence to be a “threat to humanity”. For the future, he envisions the device to be similar to a video game where the brain reverts to the last saved state.
Also Read: Who decides Pakistan poll dates, president or poll panel? The latest in Pakistan election imbroglio Neuralink evolution
The company, which is headquartered in California, was founded in 2016 by a team of eight members, including Musk and seven other scientists and engineers. It conducts research in partnership with the University of California Davis.As of 2023, only two of the original team of the eight remain with the company.In January last year, the company was reportedly criticised for its work culture of “blame and fear” and mismanagement.In February last year, Neuralink was hit with accusations that the company along with UC Davis had mistreated several monkeys and subjected them to physical and psychological distress as well as chronic and painful infections from surgeries, which was reported widely.Among 23 macaque monkeys involved in research, the complaints by the Physicians Committee for Responsible Medicine, an animal-welfare-in-research advocacy group, reportedly stated that 15 died or were euthanised, and that evidence for this was withheld.Neuralink, according to a Reuters report, stated that macaque monkeys died and were euthanised after experimentation and not during experiments. It also denied that animal abuse had […]
