Learn about brain health and nootropics to boost brain function
New Tech Could Record Deep-Brain Activity From Surface
0
Modern technology for recording deep-brain activity involves sharp metal electrodes that penetrate the tissue, causing damage that can compromise the signal and limiting how often they can be used.
A rapidly growing area in materials science and engineering is to fix the problem by designing electrodes that are softer, smaller, and flexible — safer for use inside the delicate tissues of the brain. Just last week, researchers from the University of California, San Diego, reported the development of a thin, flexible electrode that can be inserted deep within the brain and communicate with sensors on the surface.
But what if you could record detailed deep-brain activity without piercing the brain?
A team of researchers (as it happens, also from UC San Diego) have developed a thin, flexible implant that “resides on the brain’s surface” and “can infer neural activity from deeper layers,” said Duygu Kuzum, PhD , a professor of electrical and computer engineering, who led the research.
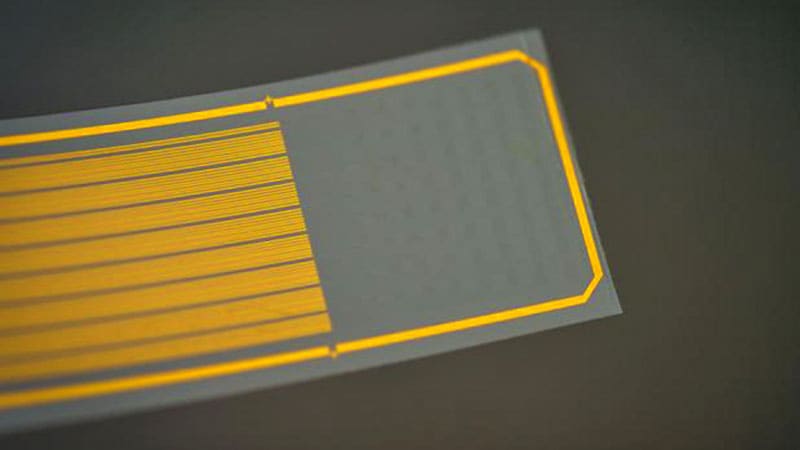
By combining electrical and optical imaging methods, and artificial intelligence, the researchers used the device — a polymer strip packed with graphene electrodes — to predict deep calcium activity from surface signals, according to a proof-of-concept study published this month in Nature Nanotechnology .
“Almost everything we know about how neurons behave in living brains comes from data collected with either electrophysiology or two-photon imaging,” said neuroscientist Joshua H. Siegle, PhD , of the Allen Institute for Neural Dynamics in Seattle , who not involved in the study. ” Until now, these two methods have rarely been used simultaneously.”
The technology, which has been tested in mice, could help advance our knowledge of how the brain works and may lead to new minimally invasive treatments for neurologic disorders. Multimodal Neurotech: The Power of 2-in-1
Electrical and optical methods for recording brain activity have been crucial in advancing neurophysiologic science, but each technique has its limits. Electrical recordings provide high “temporal resolution”; they reveal when activation is happening, but not really where. Optical imaging, on the other hand, offers high “spatial resolution,” showing which area of the brain is lighting up, but its measurements may not correspond with the activity’s timing.
Research over the past decade has explored how to combine and harness the strengths of both methods. One potential solution is to use electrodes made of transparent materials such as graphene, allowing a clear field of view for a microscope during imaging. Recently, University of Pennsylvania scientists used graphene electrodes to illuminate the neural dynamics of seizures .
But there are challenges. If graphene electrodes are very small — in this case, 20 µm in diameter — they become more resistant to the flow of electricity. Kuzum and colleagues addressed this by adding tiny platinum particles to improve electrical conductivity. Long graphene wires connect electrodes to the circuit board, but defects in graphene can interrupt the signal, so they made each wire with two layers; any defects in one wire could be hidden by the other.
By combining the two methods (microelectrode arrays and two-photon imaging), the researchers could see both when brain activity was happening and where, including in deeper layers. They discovered a correlation between electrical responses on the surface and cellular calcium activity deeper down. The team used these data to create a neural network (a type of artificial intelligence that learns to recognize patterns) that predicts deep calcium activity from surface-level readings.
The tech could help scientists study brain activity “in a way not possible with current single-function tools,” said Luyao Lu, PhD , professor of biomedical engineering at George Washington University in Washington, DC, who was not involved in the study. It could shed light on interactions between vascular and electrical activity, or explain how place cells (neurons in the hippocampus) are so efficient at creating spatial memory.
It could also pave the way for minimally invasive neural prosthetics or targeted treatments for neurologic disorders, the researchers say. Implanting the device would be a “straightforward process” similar to placing electrocorticography grids in patients with epilepsy , said Kuzum.
But first, the team plans to do more studies in animal models before testing the tech in clinical settings, Kuzum added.