Learn about brain health and nootropics to boost brain function
Living model of brain reveals how human neurons work together to process information
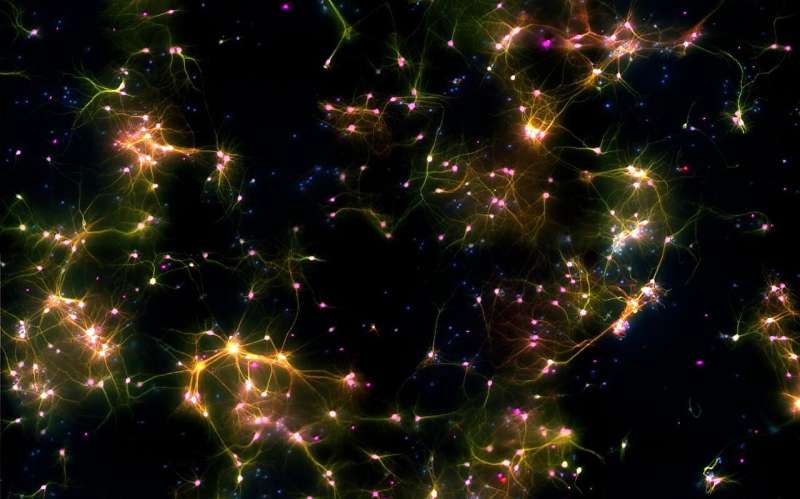
“Dishbrain’ under the microscope. A microscopy image of neural cells where fluorescent markers show different types of cells. Green marks neurons and axons, purple marks neurons, red marks dendrites, and blue marks all cells. Where multiple markers are present, colors are merged and typically appear as yellow or pink depending on the proportion of markers. Credit: Cortical Labs A paper published in Nature Communications shows that when neurons are given information about the changing world around them (task-related sensory input) it changes how they behave, putting them on edge so that tiny inputs can then set off “avalanches” of brain activity, supporting a theory known as the critical brain hypothesis.
The researchers, from Cortical Labs and the University of Melbourne, used DishBrain, a collection of 800,000 human neural cells learning to play Pong.
It is the strongest evidence to date in support of a controversial theory of how the human brain processes information.
According to the critical brain hypothesis, big complex behaviors are only made possible when neurons are so on edge that tiny inputs can set off “avalanches” of brain activity .
This fine balanced state is known as a “neural critical” state, and lies between two extremes—the runaway excitation seen in disorders such as epilepsy, and a coma state where signals stall.
“It not only shows the network reorganizing into a near-critical state as it is fed structured information but that reaching that state also leads to better task performance,” says Dr. Brett Kagan, Chief Scientific Officer of biotech start-up Cortical Labs, which created DishBrain.
“The results are astonishing, way beyond what we thought we would achieve.”
The research adds a vital piece to the puzzle of the critical brain hypothesis.
Until now, there has been little experimental evidence demonstrating whether criticality is a general feature of biological neuronal networks or whether it is related to informational load.
“Our results suggest that near-critical network behavior emerges when the neural network is engaged in a task but not when left unstimulated,” says Dr. Kagan.
However, Dr. Kagan’s research shows that criticality alone is insufficient to drive learning by a neural network . “Learning requires a feedback loop , where the network is given additional information about the consequences of an action,” says Dr. Kagan.
The latest research underlines the potential for DishBrain to help unlock the secrets of the human brain and how it works, that is not possible with animal models.
“Usually to study the brain, especially on the scale of neurons, researchers have to use animal models, but in doing so, there are lots of difficulties and one can only have a limited number of subjects,” says first author Dr. Forough Habibollahi, a research fellow at Cortical Labs.
“So when I saw DishBrain’s unique ability to answer different types of questions in a way nobody else could, I was super excited to start this project and join the team.”
Doctors also see great potential for the research to help discover treatments for crippling brain diseases.
“The DishBrain criticality project has been an amazing collaborative experience between Cortical Labs, Biomedical Engineering and Neurology,” says paper author Dr. Chris French, leader of the Neural Dynamics Laboratory at the University of Melbourne’s Department of Medicine.
“The critical dynamics of the DishBrain neurons should provide key biomarkers for diagnosis and treatment of a range of neurological diseases from epilepsy to dementia,” he says.
By building a living model brain, scientists will be able to experiment using real brain function rather than flawed analogous models like a computer to not only explore brain function but to test how drugs affect it.
The research also has the potential to solve challenges facing brain-computer interfaces that could restore functions lost as a result of neural damage, says Professor Anthony Burkitt, an author on the paper and Chair of Bio Signals and Bio-Systems of the University of Melbourne’s Biomedical Engineering Department.
“A key feature of the next generation of neural prostheses and brain-computer interfaces that we currently researching involves utilizing real-time closed loop strategies,” he says. “So the results of this study could have important implications for understanding how these control and stimulation strategies interact with the neural circuits in the brain.”
“This field of biological brain modeling is in its infancy but opens the way for a whole new area of science,” Dr. Kagan says.
Provided by University of Melbourne
Read more at medicalxpress.com