Learn about brain health and nootropics to boost brain function
Role of Peripheral Immune Cells-Mediated Inflammation on the Process of Neurodegenerative Diseases
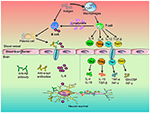
Introduction
Neurodegenerative disease is the progressive dysfunction and loss of neurons in the central nervous system (CNS), including Alzheimer’s disease (AD), Parkinson’s disease (PD) and Multiple Sclerosis (MS) ( 1 ). The mechanisms underlying their progressive nature remain unknown. To date, aging and immunity are closely associated with the pathogenesis of neurodegenerative diseases. Immunosenescence refers to the gradual deterioration of the immune system brought on by natural age advancement. It involves both the host’s capacity to respond to infections and the development of long-term immune memory, which could accelerate the progression of neurodegenerative diseases ( 2 ).
Despite different triggering events, a common feature is brain inflammation ( 3 ). It is clear that neuroinflammation during compensatory period is beneficial, which help combat infections, promote tissue repair, remove necrotic cells, shape the brain during development and repair following damage. Upon decompensatory period, a vicious cycle of glial priming and release of pro-inflammatory factors promote neuronal damage ( 4 ). On the other hand, chronic inflammation, including chronic intestinal inflammation, diabetes, obesity, and systemic lupus erythema, could cause cognitive impairment, learning and memory deficits, and human depression ( 5 , 6 ). Moreover, long-term use of non-steroidal anti-inflammatory drugs would suppress the peripheral immunity and reduce the incidence of PD by about 50% ( 7 ). In PD mice model, intraperitoneal lipopolysaccharide (LPS) injection combined with intravenous administration of two different recombinant α-synuclein (α-syn) pathogenic strains resulted in overactivation of microglia and further promoted the recruitment of leukocytes toward the brain and the spinal cord ( 8 ). Likewise, inhibiting migration of T cells or B cells into the brain rendered the CNS susceptible to devastating infections. However, the nature of peripheral immune cells in neurodegenerative diseases progression remains unclear. Thus, this review summarized the roles of peripheral immune cells on the pathological progression of neurodegenerative diseases. Roles of Peripheral Immune Cells on Neurodegenerative Diseases
Mononuclear Phagocyte System
Monocyte
Monocyte is the largest type of white blood cell in the peripheral blood that could differentiate into macrophages or dendritic cells (DCs) ( 9 ). Monocyte triggers innate immune responses by regulating Toll-like receptors (TLRs), scavenger receptors, phagocytosis and complement-mediated responses. Recent studies revealed that gut dysbiosis, a primary element behind various gastrointestinal disorders, might augment LPS, pro-inflammatory factors and monocytes, thus leading to increased intestinal and blood brain barrier (BBB) permeability through microbiota-gut-brain axis. Correspondingly, accumulation of axonal damage, misfolded proteins and neuronal demyelination facilitates the pathogenesis of neurodegenerative disorders, such as AD, PD and MS ( 10 ).
In AD patients, a higher proportion of monocytes in the peripheral blood was discerned, whereas the interaction between monocytes and platelets in the blood was not altered. Besides, cathepsin D, a major lysosomal aspartic protease, was underexpressed in monocytes, causing the defective degradation of amyloid-β (Aβ) by monocytes ( 11 ). However, the sensitivity of monocytes toward Aβ peptides was decreased, indicating that there might be a critical link between the interaction of platelets and monocytes in AD ( 12 ).
Transcriptomics analysis showed that monocytes isolated from peripheral blood of PD patients conferred pro-inflammatory effects. The increase in the number of classical monocytes in PD blood and the decrease in the number of non-classical monocytes might result from the increased monocyte differentiation or increased migration from the bone marrow ( 13 ). In contrast, monocytes play an important role in repairing of the injured brain. For example, continuous low-dose injections of LPS in the periphery caused chronic inflammation and the tolerance of peripheral monocytes. Once CNS was stimulated again, dopaminergic neuronal damage was reduced ( 14 ). Of note, PD-associated gene DJ-1 deficiency attenuated monocyte infiltration into the damaged brain, which in turn led to delay in repairing of brain injury in mice ( 15 ). Furthermore, the chemotaxis and phagocytosis of aged monocytes were increased or decreased under different conditions. In neurodegeneration, an increase in the number of monocytes and functional changes observed in peripheral blood might be related to immunosenescence, but this change was more obvious in age-matched PD patients ( 16 ).
Currently, the blood monocyte counted in the early phase of MS was robustly associated with the clinical severity of MS, whereas the counts of the other blood cells were not related with MS severity ( 17 ). Moreover, various animal studies carried out that monocytes contributed to MS-associated neuroinflammation. While classically activated monocytes promoted inflammation, type II-activated monocytes could improve the progression of MS. Furtherly, antioxidant and anti-inflammatory alternatives inhibited monocyte secretion of pro-inflammatory cytokines, such as TNF-α, IL-6, and IL-1β, and also suppressed the phagocytosis of monocytes and thus slowed down the pathological process of MS ( 18 ). Macrophage
In the inflammatory lesions, macrophages are the dominant cells. Macrophages in peripheral blood can cross BBB to secrete pro-inflammatory factors in brain to further determine the survival of neurons ( 19 ). Production of these inflammatory factors in brain is generally considered to be the primary mechanisms underlying the development of neuronal damage in response to chronic inflammation ( 20 ). Additionally, the renin-angiotensin system acts on macrophages via different signaling pathways. Angiotensin (Ang) II type 1 receptors (ATR) drive pro-inflammatory macrophage responses in neuroinflammation via regulation of chemokines. Interestingly, macrophages could secrete pro-inflammatory and anti-inflammatory factors due to the autoimmune actions of inflammation ( 21 ). In CNS, microglia are the resident macrophages and play vital functions for brain development and homeostasis. The phenotypic differentiation between microglia and peripheral macrophages is verified to be age-dependent. Peripheral macrophages might express several most commonly described microglia markers in some developmental stages or pathological conditions, particularly during chronic neuroinflammation ( 22 ). At present, blood-derived macrophages are thought to contribute to brain damage and repair in yet unidentified ways ( 23 ).
A number of studies demonstrated that defects of macrophages interfered with brain clearance of Aβ, including in Aβ phagocytosis and Aβ-induced apoptosis. Macrophages derived from peripheral blood in AD patients were found to possess ineffective phagocytosis of Aβ and low resistance to apoptosis by Aβ ( 24 – 26 […]
Read more at www.frontiersin.org