Learn about brain health and nootropics to boost brain function
Searching for the Secret of How Young Blood Rejuvenates the Brain
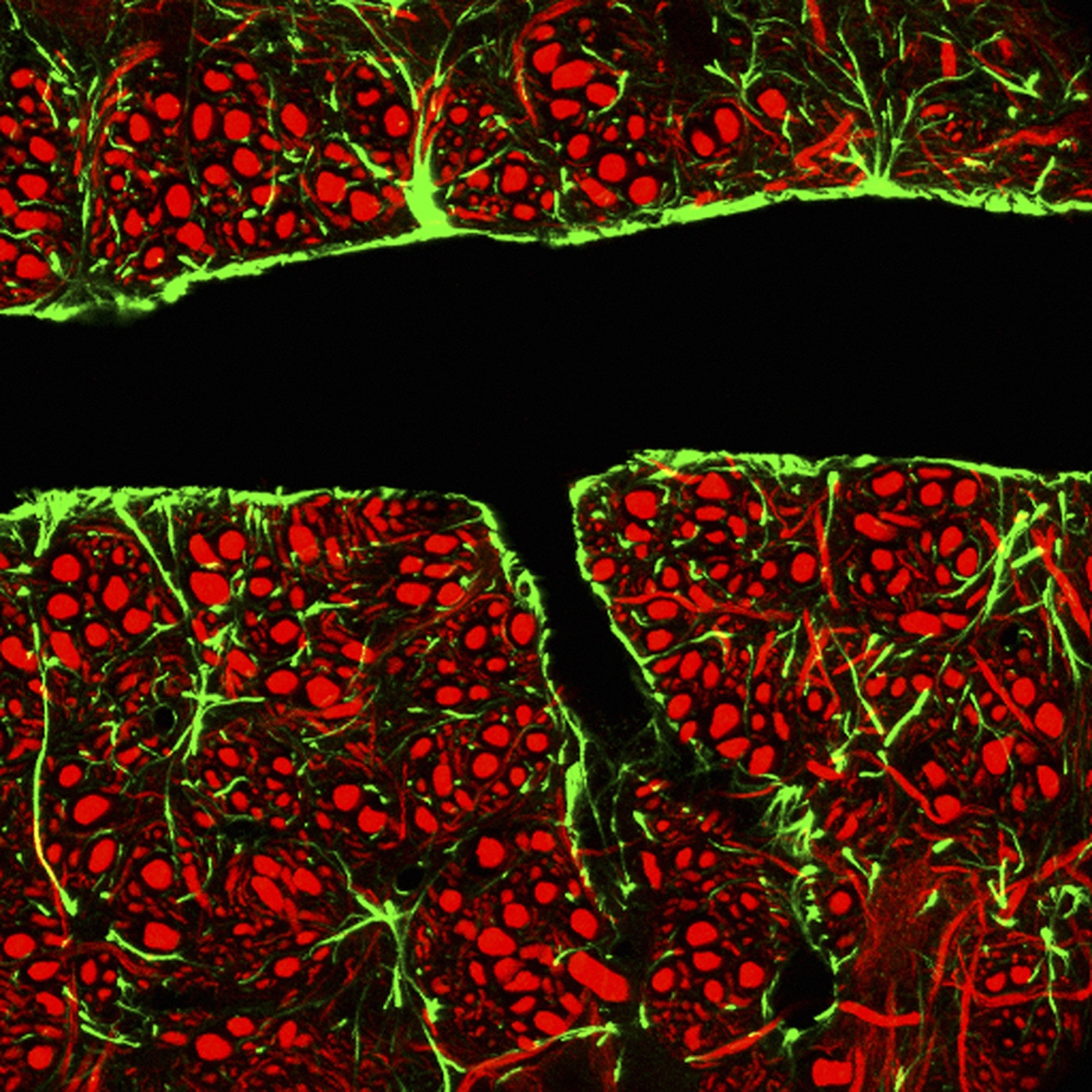
The blood brain barrier could provide a target for therapies to slow cognitive aging. Glial cells, which surround the blood vessels, are shown in green. Neurons are shown in red. Vampire legends posit that the blood of a young person can restore youth to the old, a treatment that has lured in wealthy seniors from medieval popes to Korean dictator Kim Il-Sung. But the legendary power of young blood is not simple fiction. It also appears to have a strong basis in biology.
Growing evidence over the last 20 years reveals numerous benefits. Using a procedure called parabiosis, in which two mice are joined together so that they share a circulatory system, scientists have shown that old mice that receive blood from younger animals can run faster and longer on a treadmill than their untreated counterparts. The treated mice also learn more quickly how to avoid an electrical shock and remember better how to swim through a water maze. Their brains generate new neurons in the hippocampus — the region of the brain that controls learning and memory. Young blood, it seems, is one of the most powerful anti-aging treatments — at least in mice.
Translating these findings into humans, however, is challenging. Simply transfusing blood from young donors into older people is not a viable therapeutic option: The process carries a high risk of infection and is impossible to standardize from patient to patient.
In an ideal world, scientists would isolate a specific factor in young blood that reverses the cognitive decline and memory loss that occurs with aging. Over the last decade or so, researchers have found a handful of potential candidates. But it has also become clear that no single protein is responsible for the benefits of young blood — none of these individual factors is as powerful in reversing aging as parabiosis itself. “I think we have a very rudimentary understanding at this point of how [parabiosis] works,” says Tony Wyss-Coray , a neurologist at Stanford University and an investigator with the Simons Collaboration on Plasticity and the Aging Brain (SCPAB).
Indeed, the process may involve more than simply adding a key ingredient. Some research suggests that removing factors from old blood may be just as important as adding factors from young blood. Age-related changes in the brain’s immune system and the barrier that regulates which proteins go in and out of the brain further complicate the picture. “I really think we’re not going to find some magic gene where if you fix this one system and put a Band-Aid on it, it’ll make all the difference,” says Jennifer Garrison , a molecular biologist at the Buck Institute for Research on Aging in Novato, California. Instead, the fix will require multiple proteins and other blood factors, as well as a better understanding of the body’s physiology.
Hunting for the magic protein
For a brief moment in 2014 , it seemed that researchers might have found the magic blood protein that could explain the effects of parabiosis. Lee Rubin , a stem cell biologist at Harvard University and a SCPAB investigator, and his colleagues zeroed in on a protein called GDF11, which some evidence suggests is prevalent in young blood but decreases with age. Injecting GDF11 into old mice, they found, could improve blood flow in the brain and even spark the growth of new neurons in the hippocampus.
Rubin says that GDF11 is probably one of multiple proteins in young blood that lead to rejuvenation. He and others have identified about half a dozen blood proteins that are elevated in young animals’ blood and seem to play a role in brain aging. They include TIMP2 , a protein found in umbilical cord blood, and klotho , a hormone produced in the kidneys. In some cases, injecting these proteins into old mice can enhance the growth of new neurons, regenerate muscles, and generally make the mice appear younger than their elderly counterparts.
Meanwhile, other researchers have found a number of harmful blood proteins that increase with age. Injecting them into young mice quickly causes cognitive problems and muscle degeneration. Bioengineer Irina Conboy of the University of California, Berkeley, for instance, thinks that the old factors overwhelm the young ones. In a recent paper , her group removed half of an old mouse’s blood plasma and replaced it with saline. The method, they found, was as effective at restoring brain and muscle function as replacing it with young blood. In fact, some of the proteins that are prevalent in young blood reappeared as soon as the old blood was diluted, suggesting they’d been suppressed by the old blood.
This plasma dilution system, known as plasmapheresis, is already used to treat autoimmune conditions such as multiple sclerosis and lupus, and Conboy believes a version of this treatment could be used as an anti-aging therapy as well. Earlier this year, her team started a small clinical trial in which they diluted old patients’ blood with saline, and she expects results early next year. “You can’t completely suppress [old blood proteins] because there is no such thing as a bad protein,” she says. “All proteins are evolved to do some important things in our bodies. What happens is that they just become excessive.” Old mice injected with a protein associated with exercise (right) begin producing more new neurons (green) in the hippocampus than control mice (left), suggesting this treatment mimics the beneficial cognitive effects of exercise. Credit: Horowitz et al. Science 2020. It’s not only youth that confers anti-aging power on blood. Exercise — a well-established way to protect against the cognitive effects of aging — seems to have similar benefits. In a July paper published in Science , Saul Villeda , a neuroscientist at the University of California, San Francisco and an SCPAB investigator, gave a group of sedentary old mice transfusions of blood from old mice that regularly exercised. After three weeks, the sedentary mice started performing significantly better on learning and memory tests and produced more neurons in their brains. The researchers found that […]
Read more at www.simonsfoundation.org