Learn about brain health and nootropics to boost brain function
The expectant brain: adapting for motherhood
Key Points
The expectant brain undergoes many changes to maximize the likelihood of a successful outcome of the pregnancy. These adaptations are driven by pregnancy hormones and ensure adequate nutrient supply to the fetus, protection from maternal stress hormones, appropriate organization of parturition and the delivery of maternal care.
Exposure to stress or glucocorticoids during pregnancy can adversely programme the fetuses, making them more susceptible to disease in adulthood. One protective mechanism against this effect involves endogenous-opioid inhibition of the mother’s responses to stress in pregnancy, which reduces the exposure of the fetus to maternal glucocorticoids.
Increased food intake in pregnancy is permitted by the resetting of central appetite control mechanisms, for example, the emergence of central leptin resistance. This resetting ensures sufficient nutrients for the fetus(es), extra energy for the mother, and a surplus of energy for storage as fat in preparation for lactation.
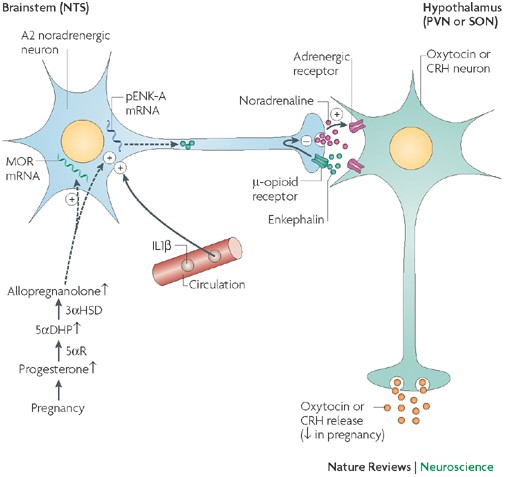
Inhibitory-opioid mechanisms prevent the premature activation of oxytocin neurons (and hence preterm birth) and aid the accumulation of neurohypophysial oxytocin stores. Allopregnanolone, a neuroactive metabolite of progesterone, restrains oxytocin neurons by enhancing the effectiveness of GABA synapses, but also induces opioid inhibition.
Dopamine neurons in the hypothalamus inhibit prolactin secretion. Before term, the stimulatory action of prolactin on these neurons is switched off, permitting increased prolactin secretion for the stimulation of lactation and maternal behaviour.
Maternal behaviour emerges rapidly after birth. This depends on ‘priming’ of the neural circuitry that organizes the components of maternal behaviour and the motivation to perform it. Priming involves the action of oestrogen, progesterone and lactogens, particularly in the medial preoptic area.
The offspring are protected from harm by a marked increase in maternal aggressiveness soon after birth. This element of maternal behaviour involves multiple neurochemical changes, in particular, increased oxytocin release and decreased activity of serotonin neurons.
In humans, withdrawal of the hormones of pregnancy might predispose women to the ‘blues’ soon after birth, and in vulnerable women might later trigger major puerperal depression.
Abstract
A successful pregnancy requires multiple adaptations of the mother’s physiology to optimize fetal growth and development, to protect the fetus from adverse programming, to provide impetus for timely parturition and to ensure that adequate maternal care is provided after parturition. Many of these adaptations are organized by the mother’s brain, predominantly through changes in neuroendocrine systems, and these changes are primarily driven by the hormones of pregnancy. By contrast, adaptations in the mother’s brain during lactation are maintained by external stimuli from the young. The changes in pregnancy are not necessarily innocuous: they may predispose the mother to post-partum mood disorders. Author information
Affiliations
> Laboratory of Neuroendocrinology, Centre for Integrative Physiology, University of Edinburgh, Hugh Robson Building, George Square, Edinburgh, EH8 9XD, Scotland, UK.
Corresponding author
Correspondence to John A. Russell . Glossary
A category of stressor (including infection and injury) that poses a real threat to homeostasis or survival and automatically activates the HPA axis. Cholecystokinin (CCK). A peptide hormone from the gastrointestinal tract that stimulates digestion and signals satiety to the brain. Interleukin-1β (IL1β). A pro-inflammatory cytokine that is produced by macrophage cells in response to infection. Endogenous opioids Peptides, including dynorphins, endomorphins, endorphins and enkephalins, that are produced naturally in the body and, like morphine, act through opioid receptors. Neuroactive steroid A steroid derivative that rapidly alters neuronal excitability through interaction with neurotransmitter-gated ion channels. Allosteric modulator A molecule that binds to a receptor at a regulatory site that is distinct from the active ligand-binding site to influence the receptor’s function. Endocannabinoid A signalling molecule that is structurally related to tetrahydrocannabinol (the main active substance found in cannabis). Endocannabinoids released by neurons act through cannabinoid receptors to modulate synaptic input. Lactogen A peptide hormone that stimulates milk production (for example, prolactin and placental lactogen). Mitral cell A type of neuron that is located in the olfactory system and that processes and transmits information from primary olfactory sensory neurons to other brain regions. Transcriptome The set of all mRNAs produced in a cell or a population of cells. About this article
Cite this article
Brunton, P., Russell, J. The expectant brain: adapting for motherhood. Nat Rev Neurosci 9, 11–25 (2008). https://doi.org/10.1038/nrn2280