Nature Knows and Psionic Success
God provides
Neuroprotectin D1 Protects Against Postoperative Delirium-Like Behavior in Aged Mice
Department of Anesthesiology, Zhongnan Hospital of Wuhan University, Wuhan, China
Postoperative delirium (POD) is the most common postoperative complication affecting elderly patients, yet the underlying mechanism is elusive, and effective therapies are lacking. The neuroinflammation hypothesis for the pathogenesis of POD has recently emerged. Accumulating evidence is supporting the role of specialized proresolving lipid mediators (SPMs) in regulating inflammation. Neuroprotectin D1 (NPD1), a novel docosahexaenoic acid (DHA)-derived lipid mediator, has shown potent immunoresolvent and neuroprotective effects in several disease models associated with inflammation. Here, using a mouse model of POD, we investigated the role of NPD1 in postoperative cognitive impairment by assessing systemic inflammatory changes, the permeability of the blood–brain barrier (BBB), neuroinflammation, and behavior in aged mice at different time points. We report that a single dose of NPD1 prophylaxis decreased the expression of tumor necrosis factor alpha TNF-α and interleukin (IL)-6 and upregulated the expression of IL-10 in peripheral blood, the hippocampus, and the prefrontal cortex. Additionally, NPD1 limited the leakage of the BBB by increasing the expression of tight junction (TJ)-associated proteins such as ZO-1, claudin-5, and occludin. NPD1 also abolished the activation of microglia and astrocytes in the hippocampus and prefrontal cortex, which is associated with improved general and memory function after surgery. In addition, NPD1 treatment modulated the inflammatory cytokine expression profile and improved the expression of the M2 marker CD206 in lipopolysaccharide (LPS)-stimulated macrophages, which may partly explain the beneficial effects of NPD1 on inflammation. Collectively, these findings shed light on the proresolving activities of NPD1 in the pro-inflammatory milieu both in vivo and in vitro and may bring a novel therapeutic approach for POD. Introduction
Postoperative delirium (POD), defined as delirium occurring mainly within 1 week after surgery, is a common neuropsychiatric complication characterized by fluctuating and concurrent disturbances of attention, cognition, psychomotor behavior, emotion, and sleep–wake rhythm ( Auerbach et al., 2018 ). POD has been linked to higher mortality, prolonged hospitalization, and an increased risk of long-term cognitive impairment ( Robinson and Eiseman, 2008 ; Inouye et al., 2014 ), and it imposes an additional medical burden on governments and society ( Inouye et al., 2014 ; Partridge et al., 2018 ). The morbidity of POD ranges from 14% in general medical units to 82% in intensive care units, with an increased prevalence in elderly patients in particular ( Bruce et al., 2006 ; Marcantonio, 2011 ; American Geriatrics Society Expert Panel on Postoperative Delirium in Older, 2015 ). With the growing aging population, the number of elderly patients who need surgery/anesthesia treatments has been increasing, as well as the prevalence of POD. However, there are no effective therapies for this complication due to the undefined underlying pathophysiology.
Recent studies highlight the importance of neuroinflammation in the development of POD ( Maclullich et al., 2008 ; Hirsch et al., 2016 ; Forsberg et al., 2017 ). Surgical trauma activates the innate immune system, leading to the systemic release of cytokines ( Hirsch et al., 2016 ). Humoral pro-inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6), have been reported to be associated with the leakage of the blood–brain barrier (BBB), which leads to the entry of pro-inflammatory cytokines and monocyte-derived macrophages, resulting in the activation of glia, including microglia and astroglia ( Terrando et al., 2011 ; Hu et al., 2018 ). The interaction between the peripheral and central immune systems amplifies inflammation in the brain ( D’Mello et al., 2009 ; Perry and Teeling, 2013 ), and the cascade of neuroinflammation induces synaptic dysfunction and neuronal apoptosis, which ultimately impairs cognitive function ( Munster et al., 2011 ; Plaschke et al., 2016 ; Skvarc et al., 2018 ). On this basis, treatments targeting the regulation of neuroinflammation show great potential as candidate therapies for POD.
Along with passive termination of inflammation, POD resolution actively participates in the restoration of acute inflammation as a coordinated process, which is regulated by specialized proresolving lipid mediators (SPMs; Serhan et al., 2014 ). SPMs are endogenously biosynthesized from essential fatty acids with potent anti-inflammatory and immunoregulatory properties ( Serhan et al., 2002 ; Hong et al., 2003 ). Protectin D (PD), which is known as neuroprotectin D1 (NPD1) when synthesized in the neural system, is one of the SPMs derived from omega-3-polyunsaturated fatty acid docosahexaenoic acid (DHA). NPD1 shares biological activities with other lipid mediators such as resolvins and maresins, including accelerating nonphlogistic macrophage phagocytosis, inhibiting neutrophil infiltration, and regulating the production of cytokines and chemokines ( Serhan et al., 2002 ; Mukherjee et al., 2004 ; Hong et al., 2014 ). Additionally, NPD1 has been demonstrated to be neuroprotective in preclinical models of Alzheimer’s disease, which shares some characteristics with POD, such as memory impairment ( Lukiw et al., 2005 ; Safavynia and Goldstein, 2019 ).
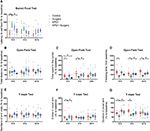
Based on these discoveries, we proposed the hypothesis that prophylaxis with NPD1 could improve cognitive behavior in a POD model of laparotomy in aged mice through its proresolving effect on inflammation induced by surgical trauma. To validate this hypothesis, we assessed the natural and learned behaviors of aged mice with or without NPD1 pretreatment and the inflammation events both in the periphery and in the central nervous system (CNS). Furthermore, we aimed to determine whether NPD1 exerts anti-inflammatory and proresolving properties by promoting macrophage polarization, which is pivotal in promoting the restorative process in acute inflammation. Materials and Methods
Animals
The experimental protocol was approved by the Animal Ethics Committee of Zhongnan Hospital of Wuhan University, and all experiments were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Female C57BL/6 mice (Changsha Tianqin Biotechnology Company Limited, Changsha, China; 18 months old and weighing 30–40 g) were group-housed with four to five mice per cage under a 12-h light/dark cycle in a temperature-controlled (25 ± 2°C) room with free access to standard rodent water and food. Surgical Model
The mice were randomly divided into the control group, surgery group, NPD1 group, or NPD1+surgery group. NPD1 (Cayman Chemical, […]
Read more at www.frontiersin.org
