Learn about brain health and nootropics to boost brain function
Plasma Aβ42 and Total Tau Predict Cognitive Decline in Amnestic Mild Cognitive Impairment
Abstract
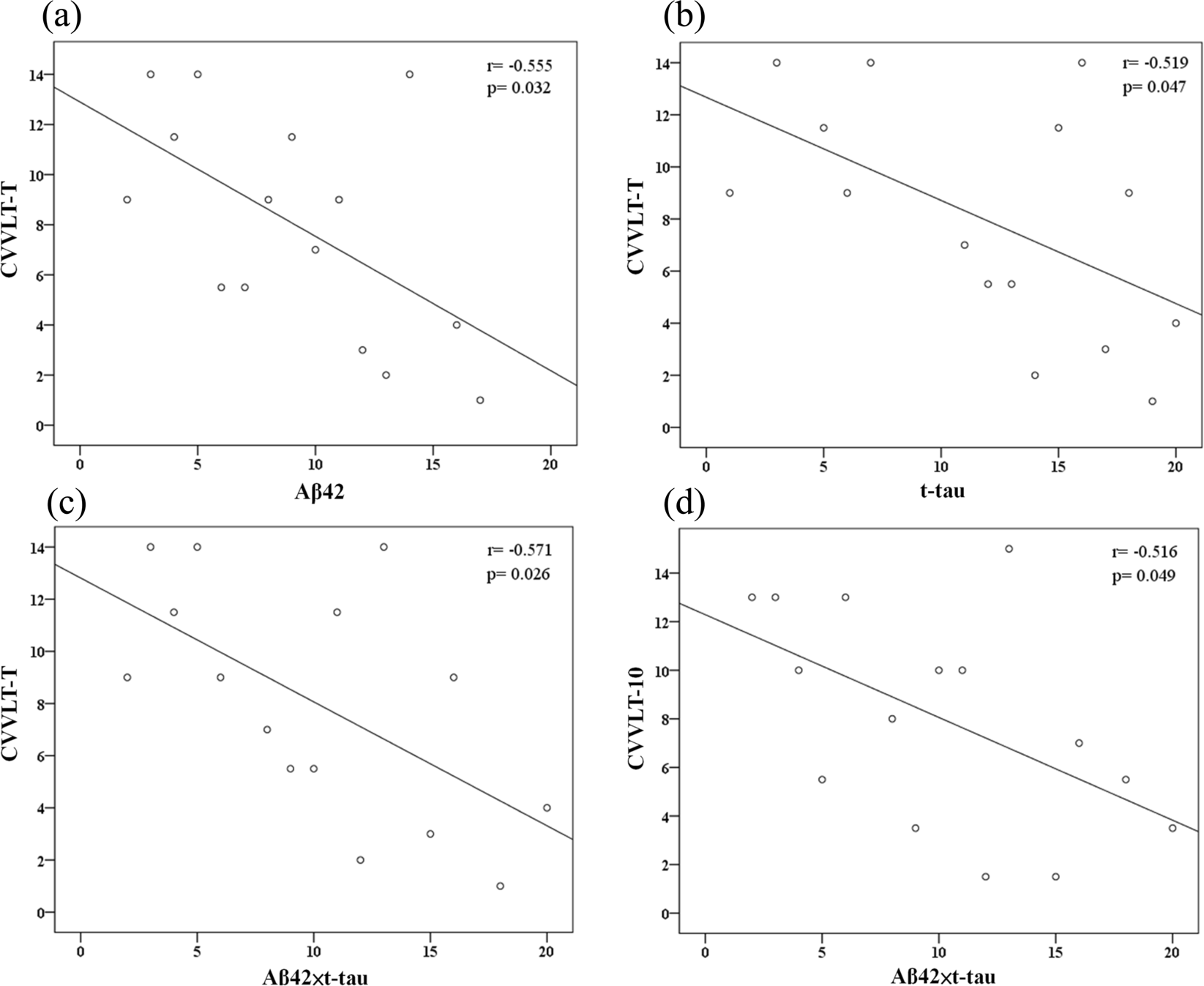
Levels of amyloid-β (Aβ) and tau peptides in brain have been associated with Alzheimer disease (AD). The current study investigated the abilities of plasma Aβ42 and total-tau (t-tau) levels in predicting cognitive decline in subjects with amnestic mild cognitive impairment (MCI). Plasma Aβ42 and t-tau levels were quantified in 22 participants with amnestic MCI through immunomagnetic reduction (IMR) assay at baseline. The cognitive performance of participants was measured through neuropsychological tests at baseline and annual follow-up (average follow-up period of 1.5 years). The predictive value of plasma Aβ42 and t-tau for cognitive status was evaluated. We found that higher levels of Aβ42 and t-tau are associated with lower episodic verbal memory performance at baseline and cognitive decline over the course of follow-up. While Aβ42 or t-tau alone had moderate-to-high discriminatory value in the identification of future cognitive decline, the product of Aβ42 and t-tau offered greater differential value. These preliminary results might suggest that high levels of plasma Aβ42 and t-tau in amnestic MCI are associated with later cognitive decline. A further replication with a larger sample over a longer time period to validate and determine their long-term predictive value is warranted.
Introduction
Mild cognitive impairment (MCI) refers to a transitional state on the continuum of cognitive function between normal aging and mild dementia. Amnestic MCI, characterized by an isolated deficit in episodic memory accompanied by intact general cognitive functioning, has been associated with biomarkers for Alzheimer disease (AD) and is now recognized as a risk factor for AD1,2,3,4. Early identification of subsequent cognitive decline in MCI patients is critical for prompt clinical intervention and therapeutic options. Thus, seeking validated biomarkers for risk of cognitive decline is crucial.
To date, validated biomarkers associated with AD include measurement of brain Aβ deposition presented by Aβ42 levels in cerebrospinal fluid (CSF), cortical amyloidosis on positron emission tomography (PET), measures of AD-typical neurodegeneration indicated by total-tau (t-tau) and phosphorylated-tau levels in CSF, hypometabolism on PET, and cerebral atrophy on magnetic resonance imaging (MRI)5. Increasing evidence supports an ordered, sequential, non-paralleled temporal trajectory of these five central biomarkers over many years in the continuum of AD6,7. In addition, these biomarkers play significant roles in the definition of AD pathology5,8. However, many obstacles, including invasiveness, time, cost, and accessibility to health care services, limit the widespread use of these biomarkers in the clinical setting. Rapid, minimally-invasive, and low-cost blood-based biomarkers are needed to assist in the identification of underlying pathophysiological processes involved in the early stages of AD and in longitudinal tracking of various disease indicators of progression of AD pathology.
Aβ accumulation and neurofibrillary tangle formation of phosphorylated-tau precedes full-blown clinical symptoms of AD by many years to decades, while Aβ deposition plateaus when patients progress into the clinical MCI phase of AD9. At this stage of the disease, neurofibrillary tangle formation, increasing gliosis, and progressive neuronal loss are initiated and continue to progress into the clinical state of overt dementia9. Accumulating evidence supports that various clearance mechanisms of toxic aggregation of misfolded peptides from the brain to the blood account for a determinate amount of Aβ and tau in the blood, and therefore, the measurability of low levels of these peptides is only attainable with highly sensitive, robust, and accurate assays10,11,12. Recent researches using ultrasensitive bioanalytic techniques have demonstrated that peripheral and central markers of amyloidosis and neurodegeneration are closely correlated12,13,14,15. Newly-developing analytic technologies may provide increasing clinical access to simple, convenient, and accurate blood-based biomarkers for high-risk individuals.
Immunomagnetic reduction (IMR) assay is a relatively new high-sensitivity detection technology for the quantification of Aβ and tau. IMR involves antibody-functionalized magnetic nanoparticles in biofluids to measure the reduction in magnetic signals through use of an ultra-high-sensitivity magneto-susceptometer, a superconducting quantum interference device (SQUID)16. Previous SQUID-based IMR studies have found that the utilization of a combination of plasma Aβ42 and total tau (t-tau) levels can reliably and accurately identify AD patients in both the prodromal and dementia stage16,17,18. However, the plasma concentrations of Aβ42 and t-tau in patients with amnestic MCI have not yet been thoroughly characterized using IMR assays.
The current study used IMR to investigate the predictive value of plasma levels of Aβ42 and t-tau in cognitive decline in subjects with amnestic MCI.
Methods
The current prospective case-control study was conducted from 2015 to 2017. Study participants were consecutively recruited from the memory clinics at Taipei Veterans General Hospital and Shuang Ho Hospital in Taiwan. The inclusion criteria were age of ≥60 years and consent to a longitudinal follow-up period of clinical, neuropsychological, and brain MRI examinations. Prior to testing, written informed consent was obtained from all participants (or their legal guardians) for publication of this research article. The local institutional review board and the ethics committee of Taipei Veterans General Hospital and Shuang Ho Hospital approved the data collection protocol. We confirm that all methods were performed in accordance with the relevant guidelines and regulations by including a statement in the methods section to this effect. All authors have approved the manuscript for submission and gave consent for publication.
All participants were subjected to a standard battery of clinical and comprehensive neuropsychological assessments at baseline and during annual follow-ups. The Mini-Mental Screening Examination (MMSE)19 and the Clinical Dementia Rating (CDR)20 were used to measure global cognition and severity of dementia, respectively. The Chinese Version Verbal Learning Test (CVVLT) (9 items; total correct trials 1–4, and 10-min delayed free recall)21 was administered to evaluate verbal memory performance. Total recall (i.e., the total number of items remembered over 4 trials, CVVLT-T) and free delayed recall (i.e., 10-minute delayed free recall, CVVLT-10) were analysed.
Clinical diagnosis was based on physical examination, clinical interview, and neuropsychological assessment. A diagnosis of amnestic single-domain MCI was based on the criteria recommended by the National Institute on Aging/Alzheimer’s Association (NIA-AA) workgroups in 201122. Episodic memory impairment was determined by a score of 1.5 standard deviations below the age- and education-matched normative mean with no accompanying impairments in social or occupational functioning, as assessed by the activities of daily living scale and the instrumental activities of daily living scale. In addition, all MCI subjects had a CDR score of 0.5.
A formal cognitive test was conducted at annual follow-up visits to ascertain the development of dementia. A diagnosis of probable AD was made when patients fulfilled the core clinical criteria proposed by the NIA-AA workgroup and had a CDR score of 123. Neurological examinations, laboratory tests, and MRI were performed at baseline to exclude non-AD causes of dementia. Exclusion criteria included evidence of other neurological, psychiatric or systemic conditions that may cause cognitive impairment, such as frontotemporal dementia, dementia with Lewy bodies, stroke, vascular dementia, Parkinson’s disease, thyroid dysfunction, renal insufficiency, vitamin B12 deficiency, neurosyphilis, alcoholism, and major depression.
The MMSE score was used to measure disease progression. Subjects were dichotomized into a stable group and a declined group according to change in MMSE scores from baseline to follow-up evaluation. Patients with a decrease in MMSE score from baseline were placed in the declined group, while those with no change or an increase in MMSE score were placed in the stable group.
Venipuncture was used to collect whole blood into ethylenediaminetetraacetic acid (EDTA)-treated tubes after overnight fasting. Blood was centrifuged (1500–2500 × g, room temperature, 15 min) and the supernatant was collected and divided into various aliquots which were maintained at −80 °C until the day of testing to avoid multiple freeze/thaw cycles. Frozen plasma samples were delivered on dry ice to MagQu Co., Ltd. (New Taipei City, Taiwan) for IMR assay processing. Assays were performed without knowledge of individual identification or diagnosis.
Apolipoprotein E (ApoE) genotype was determined for all participants by polymerase chain reaction amplification and restriction enzyme digestion following methods described previously24.
The technical information and the validation accuracy of the IMR assay have been previously well described17,18,25,26,27. The selection of antibodies conjugated to the IMR reagents (MagQu Co. Ltd.; catalogue numbers: MF-AB2-0060 and MF-TAU-0060) was based on epitopes, affinity to antigens, ability to conjugate onto MagQu magnetic nanobeads, and ability to provide linearity of standard curves quantified by magnetic signal reduction. For the Aβ42 assay, 60-μl reagent (MF-AB2-0060, MagQu) was mixed with 60-μl plasma at room temperature. For the t-tau assay, 80-μl reagent (MF-TAU-0060, MagQu) was mixed with 40-μl plasma. A SQUID-based alternative current magnetosusceptometer (model XacPro-S, MagQu Co., New Taipei City, Taiwan) was used for analysis. The magnetosusceptometer detects magnetic signal changes during the course of antigen and antibody interactions, expressed as percentage reduction of immunomagnetic signals (IMR %), which are then converted to sample concentrations using values from the standard curves of the respective analytes. The reduction of oscillation detected by SQUID corresponds to the amount of analytes bound to the antibodies. Duplicates were conducted for each biomarker assay.
All statistical analyses were performed in SPSS version 22.0 for Windows (SPSS Inc., Chicago, IL). A p-value < 0.05 was considered significant. All variables were analysed through non-parametric methods. For continuous variables, differences between the stable group and the declined group were detected with a Mann-Whitney U test. For categorical variables, the Chi-square test was used. Spearman’s rank correlation coefficient was used to explore the correlation between plasma biomarker levels and cognitive performance (i.e., MMSE and CVVLT). Receiver operating characteristic (ROC) analyses were computed to identify possible useful cut-off points of single Aβ42 or t-tau analytes, or their combinations (ratio or product) to further characterize discriminatory properties between the stable and declined groups. Additional analysis with Cox proportional regression (enter method) was carried out to investigate the power of biomarker levels and respective cut-off values in the prediction of cognitive decline in MCI, in terms of hazard ratios, with and without adjusting by age, gender, years of education, and ApoE ɛ4 carrier status.
Click here to view full article