Learn about brain health and nootropics to boost brain function
Study Identifies Cytokines That Predict Cognitive Trajectories in Older Adults
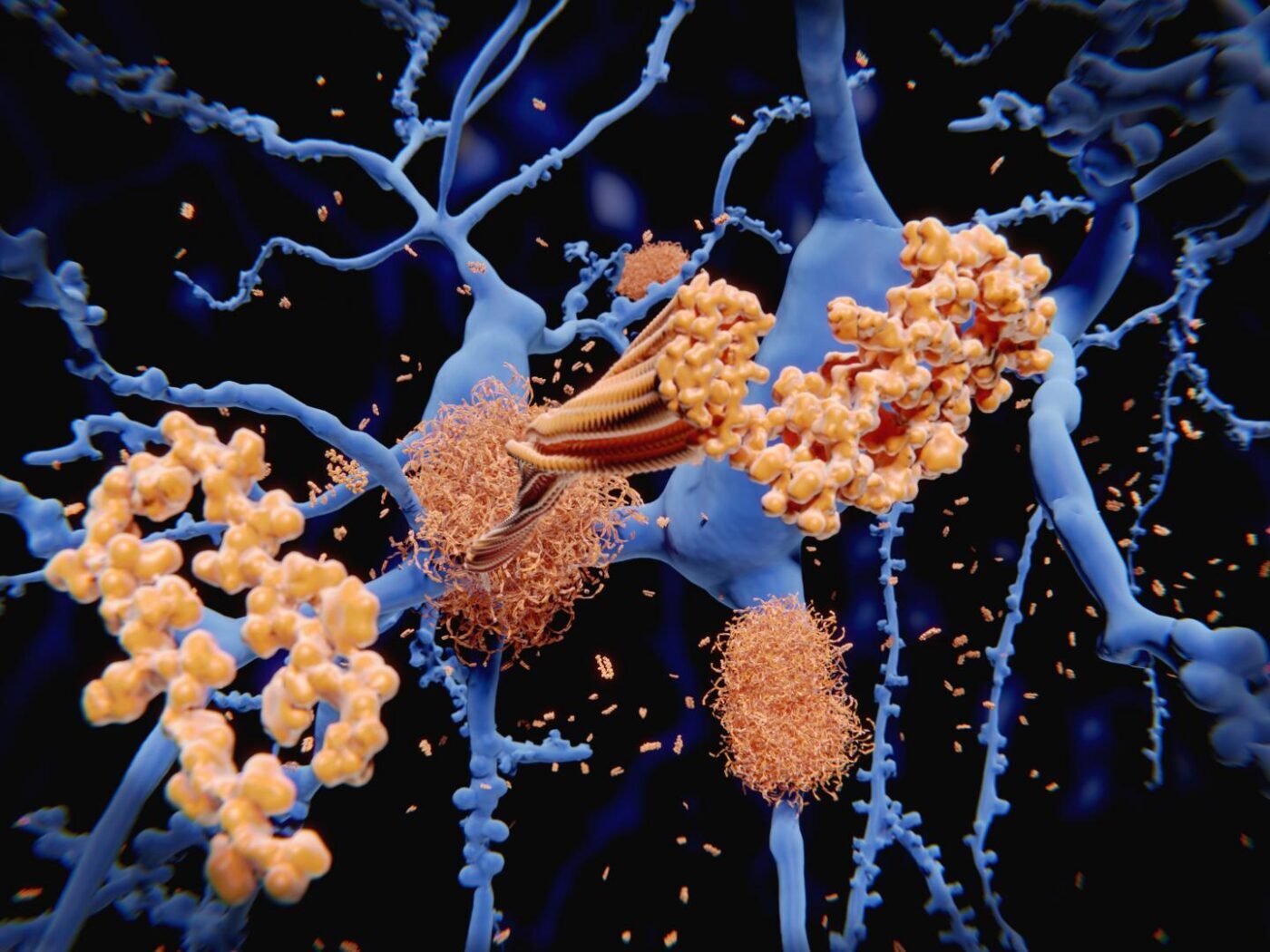
Decades before patients with Alzheimer’s disease (AD) exhibit symptoms of cognitive decline and loss of memory, their brains begin to harbor deposits of amyloid plaques and tau tangles. These deposits increase neuroinflammation in the brain that leads to neuronal cell death.
“There has always been the question whether inflammation outside the brain could contribute to the neuroinflammation in the brain that is killing nerve cells,” says Rudolph Tanzi, PhD, vice chair of neurology, co-director of the Henry and Allison McCance Center for Brain Health at Massachusetts General Hospital (MGH), and the Joseph P. and Rose F. Kennedy professor of neurology at Harvard Medical School (HMS).
In a counterintuitive finding, scientists now show elevated levels of two chemical mediators (cytokines) that enhance inflammation are associated with slower cognitive decline in normal older adults.
“These are totally unexpected results,” says Tanzi, co-senior author on the study.
The findings published in the featured article, “ Plasma IL-12/IFN-γ axis predicts cognitive trajectories in cognitively unimpaired older adults ” in the journal Alzheimer’s & Dementia, could one day be used to identify healthy people who are at risk for the devastating neurological condition before they show any symptoms.
In 2008, Tanzi’s team identified CD33, the first AD gene associated with the immune system. Yet, the role of the immune system in the earliest stage of AD when amyloid plaques and tau tangles silently begin to be deposited in the brain without any measurable cognitive symptoms, has remained unclear.
In the new study, Tanzi and his team collaborated with Harvard Aging Brain Study (HABS) investigators to find out if measuring cytokines in the blood could help predict which healthy individuals would experience cognitive decline later.
“We were studying normal elderly who were being tracked for 6 years. They were having their cognition clinically assessed and their brains imaged to determine whether they were developing the plaques and tangles that cause AD,” says Tanzi. Rudolph Tanzi, PhD, is vice chair of neurology, co-director of the Henry and Allison McCance Center for Brain Health at Massachusetts General Hospital (MGH), and the Joseph P. and Rose F. Kennedy professor of neurology at Harvard Medical School [HMS] “We wanted to know why some people have amyloid in their brain and don’t seem to be affected, while other people experience cognitive decline,” says co-senior author Jasmeer Chhatwal, MD, PhD, a neurologist at MGH, a HABS co-investigator and an assistant professor of Neurology at HMS. The partnership between the McCance Center and HABS, which is co-led by Reisa Sperling, MD, and Keith Johnson, MD, “was a natural fit,” says Chhatwal, since both groups seek to understand the secrets of healthy aging and identify biomarkers of brain health.
The study included 298 men and women from HABS, between ages 50 and 90 who had normal cognitive abilities when they volunteered and underwent retesting annually. All participants had blood drawn for cytokine assays and underwent positron emission tomography (PET) brain-imaging. Each participant’s blood was tested for nine cytokines to see if any were associated with the rate of cognitive decline and changes in the brain. The researchers use computational algorithms to correlate the cytokine data with neuropsychological and neuroimaging results.
“We expected that those who have elevated proinflammatory cytokines outside the brain, in the blood, will have more inflammation in the brain and worse cognitive decline over the 6-year period that we were tracking these normal elderly. Instead, the results were opposite. Over the six years people with amyloid in their brains go downhill more than people without amyloid in the brain–this is expected,” says Tanzi. “People who had high cytokine IL12 in the blood, even though they had the same amount of amyloid to begin with, hardly went downhill at all! That was surprising. And likewise, people who had high IFNγ, even though that is a proinflammatory cytokine, were also associated with slower cognitive decline. And that was the case whether you had amyloid in the brain or not.”
Hyun-Sik Yang, MD, neurologist at Brigham and Women’s Hospital, HABS co-investigator, assistant professor of Neurology at HMS and first author on the paper says, “Men and women with elevated levels of amyloid declined more if they had a lower value of IL-12.” High levels of IL-12 were also associated with fewer tau tangles, the study shows.
“It is counterintuitive that having higher levels of these two proinflammatory cytokines led to a better cognitive trajectory in normal elderly over 6 years, including those who had the beginnings of Alzheimer’s pathology with plaques and tangles in the brain,” says Tanzi. However, Tanzi and his team have a hypothesis that could explain these counterintuitive results.
“Over the last 10 years or so my lab has championed the antimicrobial protection hypothesis of Alzheimer’s,” says Tanzi. “My colleague Rob Moir–who passed away tragically about a year ago with glioblastoma—and I came up with this hypothesis that the amyloid in the brain can actually protect the brain against infection. If a bacteria or virus gets into the brain, amyloid quickly forms around it to trap it and protect the cells. It works as an antimicrobial peptide. This was very surprising for the field because the amyloid beta protein was always thought to be toxic junk. We thought it actually plays a role in the brain–that it is protecting the brain against infection.” Robert Moir, PhD, was a neurobiologist at MGH and Harvard University heading a research lab at the Genetics and Aging Research Unit.
This would indicate that with age, as the immune system starts to go downhill and the blood brain barrier becomes more permeable, more microbes could get into the brain, triggering amyloid, tangles and neuroinflammation—the hallmarks of Alzheimer’s pathology.
IL-12 and IFNγ are cytokines that regulate immune defense against infection via T-cells and pathogen engulfing macrophages.
“If you have an infection, T cells have to activate the macrophages to fight that infection. The two cytokines that are used for the T cells to activate the macrophages to fight infection are exactly the two cytokines that are increased,” says Tanzi.
Tanzi and his colleagues therefore hypothesize that even though the heightened cytokines are […]
Read more at www.genengnews.com